 |
 |
 |
| |
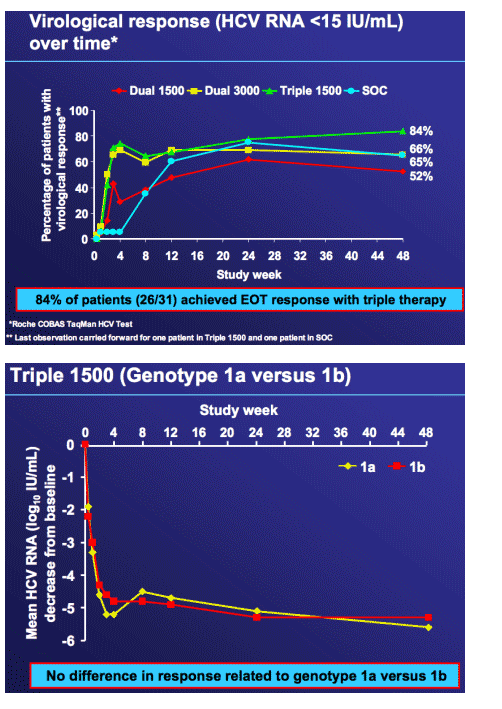
High end-of-treatment response (84%) after 4 weeks of R1626, peginterferon alfa-2a (40KD) and ribavirin followed by a further 44 weeks of peginterferon alfa-2a and ribavirin
|
| |
| |
Reported by Jules Levin
43rd EASL Conference, April 23-27, 2008, Milan, Italy
David Nelson, Paul J. Pockros, Eliot Godofsky, Maribel Rodriguez-Torres, Greg Everson, Michael W. Fried, Reem H. Ghalib, Stephen A. Harrison, Lisa M. Nyberg, Mitchell L. Shiffman, Anna Chan, George Z. Hill
AUTHOR CONCLUSIONS
Robust antiviral response after 4 weeks of R1626-based triple therapy which can be maintained through EOT at week 48
-- 84% patients with undetectable HCV RNA by end of treatment
(R1626 1500 mg bid + peginterferon alfa-2a + ribavirin)
Lack of viral resistance to R1626 after 4 weeks of triple therapy with peginterferon alfa-2a and ribavirin
Neutropenia and other lab abnormalities were fully reversible and comparable to SOC after discontinuation of R1626
Dosing of R1626 needs to be optimized to balance efficacy and safety/tolerability
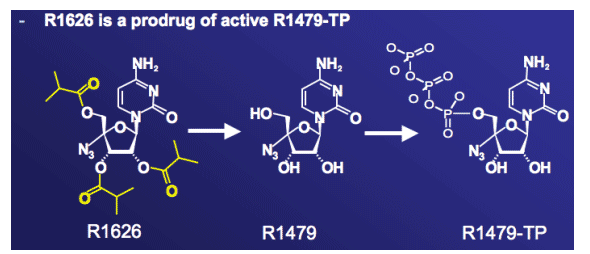
R1626: nucleoside analogue HCV polymerase inhibitor
Active against all HCV genotypes (Leveque et al. EASL poster 851)
High barrier to resistance (Le Pogam et al. EASL abstract 21)
Offers bid dosing with no food effect
Is not metabolised via cytochrome P450 enzymes
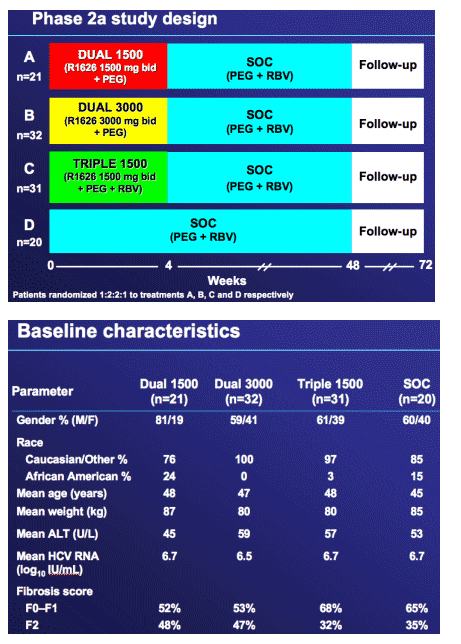
Phase 2a study objectives and design
Objective
Evaluate antiviral activity, drug resistance, and safety/tolerability of R1626 in combination with peginterferon alfa-2a (40KD) (PEG ± ribavirin (RBV) (PEGASYS + Copegu)
Design
Multi-centre, randomized, double-blind (R1626 and RBV), active controlled
4-week combination with peginterferon alfa-2a ± ribavirin with follow-up safety data on standard of care to week 48
Patient population
-- Treatment naive, genotype 1
-- Non-cirrhotic (Metavir ≦ 2, Knodell ≦ 1, Ishak ≦ 4)
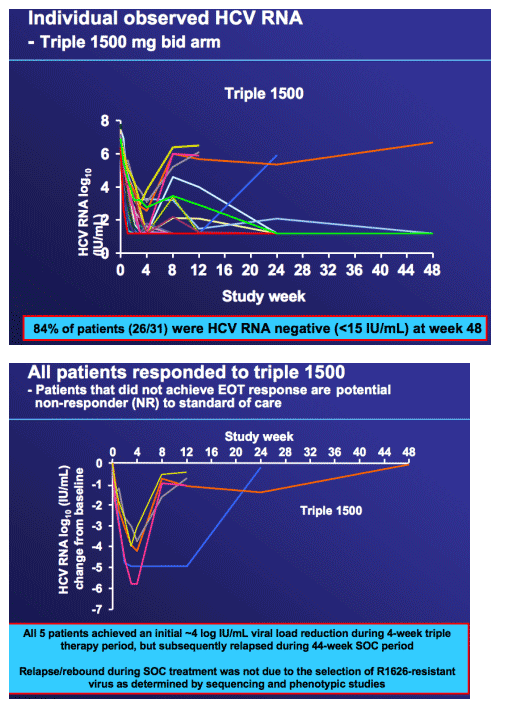
Summary of Phase 2a resistance assessment
--Phenotypic and sequence analyses at baseline and at viral rebound (³ 0.5 log10 increase above nadir)
· No viral load rebound in 4-week triple therapy
· Seven patients with viral rebound in dual arms
- All patients discontinued from R1626 prior to viral rebound
- Sequence analysis showed no known R1626-resistance mutations (S96T or N142T) at baseline or on treatment for all patients
- All on treatment samples were as sensitive as the baseline (phenotypic assay)
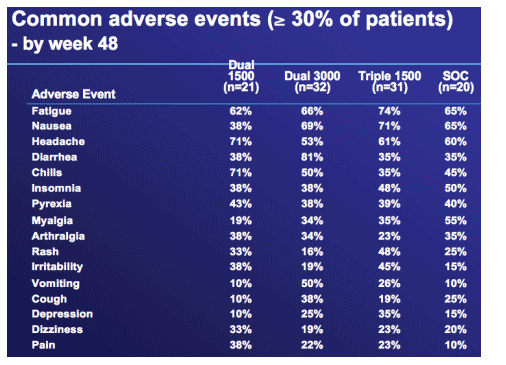
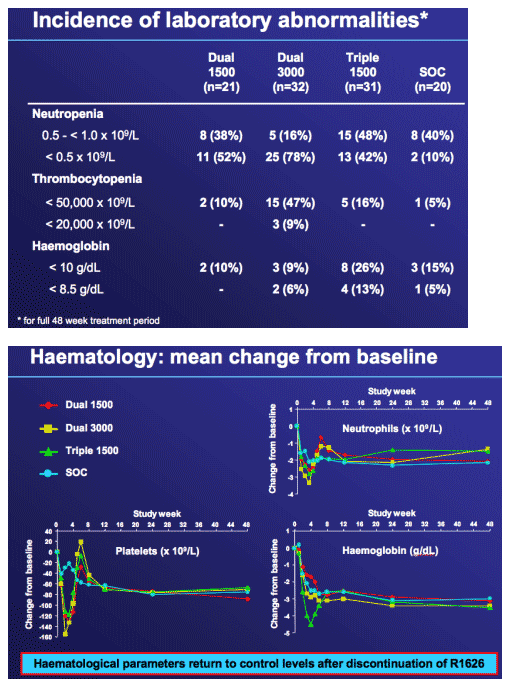
|
| |
|
 |
 |
|
|