 |
 |
 |
| |
An open-label, comparative, multicentre study of peginterferon alfa-2a (40KD) plus ribavirin in the treatment of patients chronically infected with HCV/HBV or HCV alone
|
| |
| |
Reported by Jules Levn
43rd EASL, April 2008 Milan, Italy
C-J Liu1, W-L Chuang2, C-M Lee3, S-S Wu4, L-Y Liao5, H-T Kuo6, Y-C Chao7, C-L Chen1, P-J Chen1, D-S Chen1
1National Taiwan University College of Medicine and National Taiwan University Hospital, Taiwan; 2Kaohsiung Medical University Hospital, Taiwan; 3Chang Gung Memorial Hospital, Kaohsiung, Taiwan; 4Changhua Christian Hospital, Taiwan;
5Ren-Ai Branch, Taipei City Hospital, Taiwan; 6Chi-Mei Medical Center, Taiwan; 7Tri-Service General Hospital, Taiwan
BACKGROUND
There is an urgent unmet need for efficacious therapy for patients co-infected with hepatitis B virus (HBV) and hepatitis C virus (HCV)
HBV/HCV co-infection is common in areas with a high endemic level of both infections, with a prevalence of around 7-11% in patients with chronic HBV infection and 2-10% of chronic hepatitis C patients1-3
Co-infected patients have more severe liver disease and an increased risk of hepatocellular carcinoma than mono-infected patients4,5
Few data exist on treatment of HBV/HCV co-infection. Trials assessing the treatment of co-infected patients with conventional interferon (IFN) monotherapy at conventional doses have been disappointing6,7
IFN + ribavirin (RBV) for 24 weeks in patients with HCV/HBV co-infection demonstrated an HCV sustained viral response (SVR) comparable to that observed in HCV mono-infected patients8-10
We hypothesised that when treating with peginterferon alfa-2a (PEGASYS) + RBV (COPEGUS), there will be no difference between the SVR for HCV mono-infected and HBV/HCV co-infected patients
Here we present the results of a large multicentre study with PEGASYS + COPEGUS in mono- and co-infected patients in Taiwan
AUTHOR SUMMARY
In difficult to treat HBV/HCV genotype 1 co-infected patients, 48 weeks' treatment with PEGASYS + COPEGUS achieved an HCV SVR in 72% of patients
In HBV/HCV genotype 2/3 co-infected patients, 83% of patients achieved an SVR after 24 weeks of treatment
- These results are comparable to that observed in HCV mono-infected patients where 77% of genotype 1 and 84% of genotype 2/3 infected patients achieved an SVR
HBV virologic response was obtained in 56% of patients with HBV/HCV co-infection
Importantly, HBsAg clearance - an important indicator of treatment success - was observed in 11% of HBV/HCV co-infected patients, 84% of which had undetectable HBV DNA at end of treatment
36% of HBV/HCV co-infected patients with undetectable serum HBV DNA pre-treatment experienced rebound of HBV DNA (from Jules: use of oral antiviral such as tenofovir or entecavir would suppress HBV DNA).
AUTHOR CONCLUSION
Combination therapy with PEGASYS and COPEGUS appears to be safe and effective to HBV/HCV co-infected patients with active chronic hepatitis C. The impact of the combination therapy on HBsAg clearance during longer-term observation remains to be determined.
OBJECTIVE
To evaluate the efficacy and safety of peginterferon alfa-2a (40KD) (PEGASYS) in combination with ribavirin (COPEGUS) for the treatment of patients co infected with HCV/HBV compared with those monoinfected with HCV
METHODS
· Eligible patients with active HCV (serum ALT level ≥1.5 x upper limit of normal and HCV RNA level ≥1000 copies/mL), with (n=161) or without (n=160) detectable HBsAg, were consecutively enrolled (Figure 1)
· Patients with HCV genotype 1 received 48 weeks of combination therapy with PEGASYS 180 ug weekly plus daily COPEGUS 1000-1200 mg (n=207)
· Patients with HCV genotype 2/3 received 24 weeks of combination therapy with PEGASYS 180 ug weekly plus daily COPEGUS 800 mg (n=114)
· The primary efficacy parameter was sustained HCV RNA clearance (<25 IU/mL) 24 weeks off-treatment (SVR). HCV RNA was measured using a commercial real-time PCR assay with a lower limit of detection of 25 IU/mL (Cobas Taqman HCV Test v2.0)
· Secondary endpoints were HBV viral response (<1000 copies/mL), ALT normalisation and HBsAg clearance at end of treatment and 24 weeks off treatment. HBV DNA was also measured using an in-house realtime PCR assay with a lower limit of detection of 1000 copies/mL
RESULTS
Patient characteristics
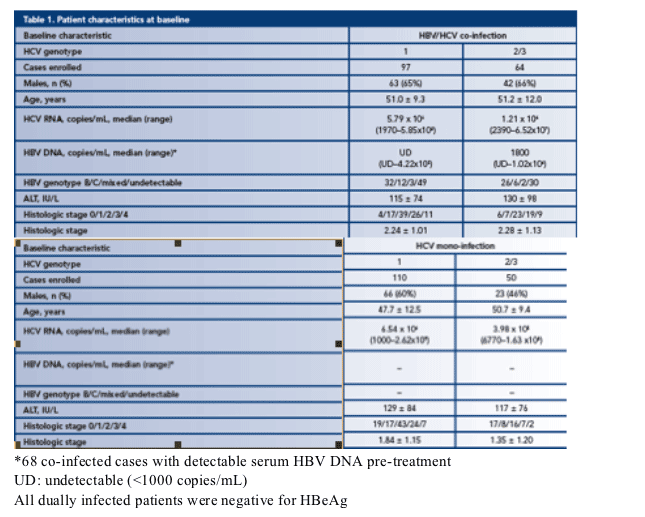
· Patient characteristics are listed in Table 1:
- Dually infected patients were negative for HBeAg
- Except in the mono-infected HCV genotype 2/3 arm, the majority of patients were male
- The mean age was 50 years but patients in the mono-infected HCV genotype 1 arm were significantly younger
- Generally, HCV RNA load was higher in genotype 1 patients than in genotype 2/3 patients
- HBV DNA level was higher in genotype 2/3 patients
HCV response
HCV genotype 2/3 infected patients had a slightly better sustained response than genotype 1 patients in both HBV/HCV co-infected and HCV mono-infected patients (Figure 2)
HCV clearance rates were comparable at the end of treatment (88% vs 92%) and 24 weeks off-treatment (83% vs 84%) for HBV/HCV co-infected and HCV mono-infected patients with genotype 2/3 infection, respectively
The majority of HCV genotype 1 infected patients with HBV/HCV co-infection achieved an SVR (72%) after 24 weeks of treatment-free follow-up and an even higher rate of HCV clearance was achieved in HCV mono-infected patients (77%)
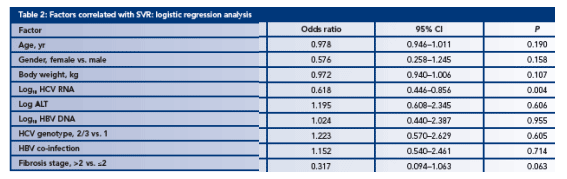
A logistic regression analysis of factors associated with achieving SVR indicates that the most significant factors are baseline HCV RNA levels and extent of liver disease (Table 2)
HBV response
Virological
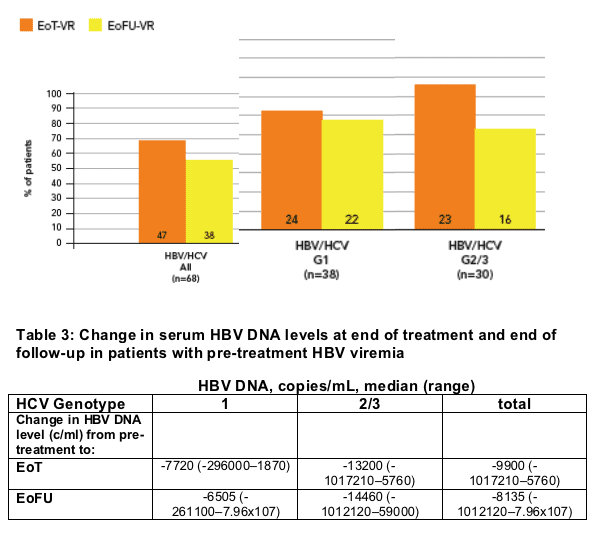
After excluding patients who withdrew, we had 145 cases with serial serum HBV DNA samples available for analysis. Of these, 68 (47%) had HBV DNA >1000 copies/mL pre-treatment
- HBV virologic response (reduction of HBV DNA to <1000 copies/mL) was obtained in 47 patients (69%) at the end of treatment and in 38 (56%) at the end of follow up (Figure 3)
Of the 77 patients with undetectable (<1000 copies/mL) serum HBV DNA pre-treatment, rebound (increase of HBV DNA to >1000 copies/mL) occurred in 28 (36%); 15/47 (31%) with genotype 1 and 13/30 (43%) with genotype 2/3.
Median HBV DNA levels at end of treatment and end of follow-up in patients with detectable HBV DNA prior to treatment are given in Table 3
Stratifying the rebound patients by presence or absence of HCV SVR, we found that the rebound rate was 37% in patients with HCV SVR and 27% in patients without HCV SVR. This difference was not significant
No rebound was associated with an elevation of serum ALT >200 IU/mL, and most of these patients had achieved
an HCV SVR
Figure 3: Viral response* (VR) at end of treatment (EoT) and 24 weeks after end of treatment (EoFU) in patients with detectable serum HBV DNA pre-treatment
* HBV virologic response, serum HBV DNA reached <1000 copies/mL at end of treatment or 24 weeks post-treatment in those with detectable serum HBV DNA pre-treatment
HBsAg clearance
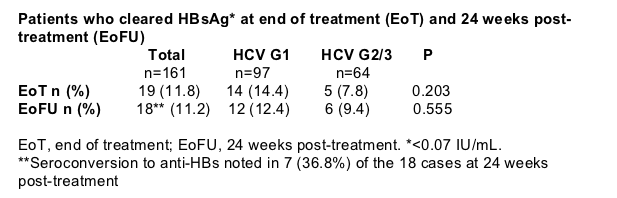
11% of HBV/HCV co-infected patients cleared HBsAg at the end of treatment-free follow-up (Table 4, Figure 6)
Baseline factors predisposing to HBsAg clearance were low HCV RNA levels, HBV genotype and low baseline HBsAg levels
Of the 85 co-infected patients with undetectable HBV prior to treatment, 12 (14%) cleared HBsAg
Of the 19 patients who cleared HBsAg, 16 (84%) had HBV DNA suppressed to undetectable levels at the end of treatment
Biochemical response (ITT)
HCV mono-infected patients had a slightly higher ALT normalisation rate than HBV/HCV co-infected patients at the end of treatment and 24 weeks post treatment (ITT analysis)
The majority (62%) of HCV genotype 1 infected patients with HBV/HCV co infection achieved a biochemical response at 24-weeks post-treatment: for non-1 GT 67% achieved normalization 24-weeks post-treatment; for monoinfection percents were a little higher: 70% for GT-1 and 78% for GT-2.
Safety
Patient withdrawals
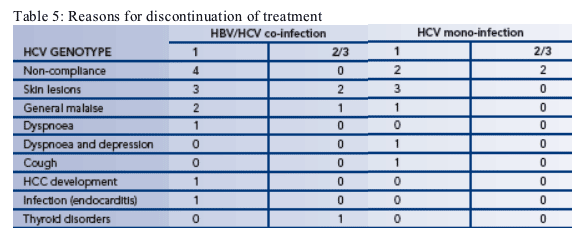
--21 patients withdrew from treatment during the study (Table 5)
-- The three most common reasons for withdrawal were non-compliance, skin lesions and general malaise
REFERENCES
1. Liu Z, Hou J. Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int J Med Sci 2006;3(2):57-62.
2. Chen DS et al. Hepatitis C virus infection in an area hyperendemic for hepatitis B and chronic liver disease: the Taiwan experience. J Infect Dis 1990;162:817 22.
3. Liu CJ, et al. Natural course and treatment of dual hepatitis B virus and hepatitis C virus infections. J Formos Med Assoc 2005;104(11):783-91.
4. Sagnelli E, et al. Virologic and clinical expressions of reciprocal inhibitory effect of hepatitis B, C, and delta viruses in patients with chronic hepatitis. Hepatology 2000;32(5):1106-10.
5. Benvegnu L, et al. Concurrent hepatitis B and C virus infection and risk of hepatocellular carcinoma in cirrhosis. A prospective study. Cancer 1994;74(9):2442-8.
6. Sagnelli E, et al. Isolated anti-HBc in chronic hepatitis C predicts a poor response to interferon treatment. J Med Virol 2001;65(4):681-7.
7. Khattab E, et al. Analysis of HCV co-infection with occult hepatitis B virus in patients undergoing IFN therapy. J Clin Virol 2005;33(2):150-7.
8. Chuang WL, et al. Viral interaction and responses in chronic hepatitis C and B coinfected patients with interferon-alpha plus ribavirin combination therapy. Antivir Ther 2005;10(1):125-33.
9. Liu CJ, et al. Ribavirin and interferon is effective for hepatitis C virus clearance in hepatitis B and C dually infected patients. Hepatology 2003;37:568-76.
10. Hung CH, et al. Combination therapy with interferon-alpha and ribavirin in patients with dual hepatitis B and hepatitis C virus infection. J Gastroenterol Hepatol 2005;20(5):727-32.
|
| |
|
 |
 |
|
|