 |
 |
 |
| |
Comparing Pegasys and PegIntron at EASL 2008 Milan
|
| |
| |
Reported by Jules Levin
There were 4 studies presented at EASL 2008 in Milan comparing Pegasys to PegIntron. The long awaited IDEAL Study results conducted by Schering Plough were presented. This study involved 3070 treatment-naive patient participants (all genotype 1) and was a 3-arm randomized, open-label, parallel-group study in 118 academic and community centers. Two doses of PegIntron were studied, 1.5 ug/kg/wk and 1.0 ug/kg/wk; the Pegasys dose was the standard 180 ug/wk. 82% of patients had >600,00 IU/mL HCV RNA. 11% had METAVIR fibrosis score of 3-4. 18% were African-American/Black.
Among genotype 1 the SVR was 41% for Pegasys, 40% for PegIntron 1.5, and 38% for PegIntron 1.0. The study authors reported relapse rates: 32% for Pegasys, 24% for PegIntron 1.5, and 20% for PegIntron 1.0. Of particular interest, patients who were 80% adherent had much higher SVR rates: 70% for PegInytron vs 61% for Pegasys. Although it was not reported clearly patients with 100% adherence would achieve even higher SVR rates.
Much data was presented so in order to get a better understanding of the study you need to review all the data presented, here is a link to that:
An Italian study (A Ascione) reported results in an oral presentation in the same session just prior to the IDEAL Study presentation. The Italian study compared Pegasys with PegIntron in a prospective, randomized, open-label, single-center trial in 320 treatment-naives. Ribavirin was dosed the same for both arms: 1000 mg/day for patients <75 kg or 1200 mg/day if >75 kg. Median HCV RNA was about 600,000 IU/ml. 66% were genotype 1 and 33-41% were genotype 2-3.
Overall SVR rate was 68% for Pegasys and 54%. For genotype 1-4, SVR was 55% for Pegasys and 40% for PegIntron.
SVR response to therapy for patients with genotype 2-3 was 88% for Pegasys and 75% for PegIntron.
For genotype 2, Pegasys SVR was 92% and 78% for PegIntron. For genotype 3, SVR was 78% for Pegasys and 71% for PegIntron. Although the differences between Pegasys and PegIntron were not statistically significant for genotype 2 and 3.
SVR for patients with high baseline viral load was 69% for Pegasys and 46% for PegIntron. For patients with low viral load the SVR was 68% for Pegasys and 65% for PegIntron.
Here is the link to a detailed study report:
There were 2 posters one from a German group and one from an Italian group reporting on studies that included a comparison between the 2 peginterferons. Here is the poster report from the Italian group reporting the PROBE Study.
PREDICTORS OF SVR IN NAIVE HCV G1 PATIENTS
IN REAL-LIFE PRACTICE: RESULTS OF THE PROBE STUDY
A. Craxi1, F. Piccinino2, A. Alberti3, A. Ascione4, M. Rizzetto5, C. Iannacone6, M. Sarracino7
1Department of Gastroenterology, University of Palermo, Italy; 2Department of Infectious Diseases, Second University of Naples, Italy; 3Venetian Institute for Molecular Medicine (VIMM), Padua,
Italy; 4Department of Gastroenterology, Hepatology Unit, Ospedale Cardarelli, Naples, Italy; 5Gastroenterology Division, University of Turin, Italy; 6Biostatistic Department Quintiles, Milan, Italy; 7Medical Affairs, Roche SpA, Monza, Italy.
One of the objectives of the PROBE (Pegylated interferons and Real Optimization of Best Efficacy) study, a large prospective analysis conducted in Italy, was to define what factors affect SVR rates in routine clinical practice. The results presented here focus on a subgroup of the PROBE study, namely patients infected with the more treatment-resistant HCV genotype 1.
METHODS
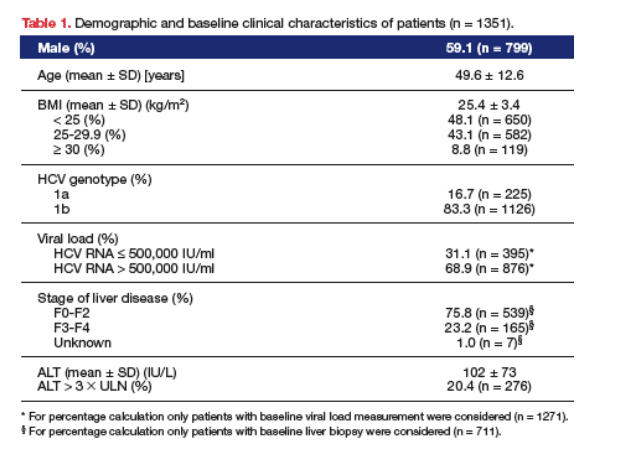
PATIENTS: the PROBE study enrolled 7445 patients with CHC from 167 hospitals in Italy. The data presented here are from 1351 patients infected with HCV genotype 1 and not previously treated with antiviral therapy.
TREATMENT: currently recommended combination therapy with pegylated interferon plus ribavirin, administered according to the protocol of each center involved, for 24 or 48 weeks. Pegylated interferon a-2a was given to 887 patients (65.7%) and pegylated interferon a-2b to 464 patients (34.3%).
EFFICACY OUTCOME: SVR rates (SVR defined as HCV RNA < 50 IU/ml, 6 months after stopping treatment).
LOGISTIC REGRESSION ANALYSIS: the variables considered by the analysis were gender, age, body mass index (BMI), baseline alanine aminotransferase (ALT), baseline viral load, HCV genotype (1a vs. 1b), cirrhosis, comorbidities, and type of pegylated interferon used (a-2a vs. a-2b).
RESULTS
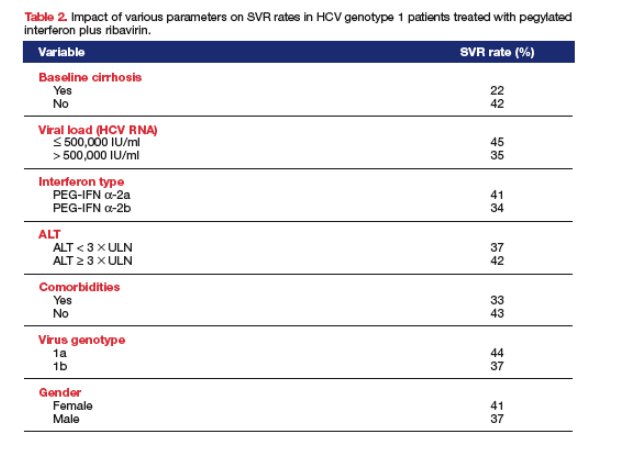
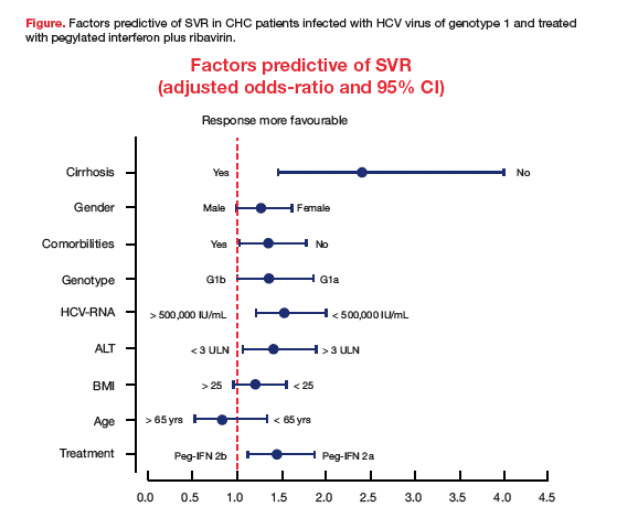
Almost one quarter (23.2%) of the non-selected, real-life HCV genotype 1 patients analyzed had histologically confirmed advanced liver disease (cirrhosis) at baseline, and over 50% of patients had a BMI ³ 25 kg/m2 (Table 1). An SVR was achieved by 38.2% of patients, which is similar to the SVR rates reported for HCV genotype 1 in registration trials. Of the variables analyzed, statistically significant independent predictors of SVR were, as expected, the absence of cirrhosis at baseline (SVR 42% vs. 22%) and a baseline viral load (HCV RNA) < 500,000 IU/ml (SVR 45% vs. 35%), but also the use of pegylated interferon a-2a (SVR 41.0% vs. 34.0%), baseline ALT ³ 3 upper limit of normal (SVR 42.0% vs. 37.0%), the absence of comorbidities, virus genotype 1a, and female gender (Table 2). The logistic regression analysis indicated factors which significantly increased the odds of achieving SVR were the absence of cirrhosis (OR 2.41, CI 95%: 1.46-3.99, p < 0.001), a baseline viral load (HCV RNA) < 500,000 IU/ml (OR 1.55, CI 95%: 1.21-1.99, p < 0.001), the use of pegylated interferon a-2a (OR 1.45, CI 95%: 1.13-1.87, p = 0.004), baseline ALT > 3 °Ñ ULN (OR 1.42, CI 95%: 1.06-1.89, p = 0.02), the absence of comorbidities (OR 1.36, CI 95%:1.04 1.78, p = 0.02), virus genotype 1a (OR 1.36, CI 95%: 1.00-1.86, p = 0.05), and female gender (OR 1.28, CI 95%: 1.00-1.62, p= 0.05) (Figure). Age and BMI were not statistically significant predictors of SVR.
The German study.
Efficacy and Tolerability of Peginterferon alfa-2a or -2b plus Ribavirin in the Routine Daily Treatment of Chronic Hepatitis C Patients in Germany: The PRACTICE Study
Witthoeft T1, Hueppe D2, John C3, Goelz J4, Meyer U5, Heyne R6, Moeller B6, Link R7, Teuber G8, Sworszt S9, Herrmann A10, Wollschlaeger A11, Baumgarten A12, Simon K-G13, Cordes J14, Moog G15, Dikopoulos N16, Mauss S17
1Medical Department I, Div. of Gastroenterology, University Hospital Schleswig-Holstein Campus Luebeck, Luebeck; 2Center of Gastroenterology, Herne; 3Center of Gastroenterology, Berlin;
4Practice Centre Kaiserdamm, Berlin; 5Center of Internal Medicine, Berlin; 6Center of Gastroenterology and Livercenter, Berlin; 7Internal Medicine, St. Josef Clinic, Offenburg; 8Johann Wolfgang Goethe University, Frankfurt/Main; 9General Practice, Gelsenkirchen; 10Friedrich Schiller University, Jena; 11Hospital Dresden Friedrichstadt, Dresden; 12General Practice, Berlin; 13Center of Gastroenterology, Leverkusen; 14Vitanus GmbH, Frankfurt/Main; 15Center of Gastroenterology, Kassel; 16Department for Internal Medicine I, University of Ulm, Ulm; 17Center for HIV and Hepatogastroenterology, Duesseldorf; all Germany
This research was supported by Roche Pharma AG, Grenzach-Wyhlen, Germany.
INTRODUCTION
The PRACTICE-study (Pegylated Interferons and Ribavirin: Analysis of CHC Treatment In Centres of Excellence) is a German nationwide retrospective observational study analysing the response to hepatitis C treatment in routine
clinical practice between 2000 and 2007.
The study included 23 gastroenterological centres with high treatment rates (>20/year) of chronic hepatitis C (CHC).
OBJECTIVE
Aim of this analysis of retrospective data is to evaluate efficacy and safety of peginterferon alfa-2a plus ribavirin (A) compared to peginterferon alfa-2b plus ribavirin (B) the treatment of cHC-patients under real life conditions.
METHODS
This evaluation is part of a large retrospective German multicentre, open-label observational study including anti-HCV positive adults with detectable HCV RNA. According to the nature of this study, selection as well as dosing and treatment duration of peginterferon and ribavirin were at the discretion of the physician.
The data set includes patients who completed treatment with peginterferon alfa-2a or alfa-2b plus ribavirin. The data collection was performed via an e-CRF online via the internet.
The documented data should refl ect the clinical routine as intended by the doctors in charge. Therefore, the statistical analysis remains descriptive.
Treatment
Patients were treated either with:
- A: Pegylated interferon alfa-2a (40KD) (Pegasys) mostly plus ribavirin (Copegus) or
- B: Pegylated interferon alfa-2b (12KD) (PegIntron) mostly plus ribavirin (Rebetol).
Matched Pairs
Matched Pairs I: To achieve comparability regarding interferon therapy, patients were matched acc. to the following criteria:
- age difference ≦3 years,
- same HCV genotype (only main type),
- same category of viral load: LVL or HVL (cut-off: ≦400.000 IU/ml),
- BMI difference ≦2 kg/m2,
- same anamnesis of hepatitis C pretreatment incl. subcategories (monotherapy, IFN-RBV-combi nation therapy, PEG-RBV-combination therapy, virological non-response, not adequate pre treatment),
- presence of substitution (yes / no),
- presence of HIV-coinfection (yes / no).
Matched Pairs II: To include ribavirin treatment, a second analysis of matched pairs was initiated: In addition to the criteria of Matched Pairs I, patients were matched acc. to:
- cumulative ribavirin dose (≦60% / >60%-80% / >80%-100% / >100%).
RESULTS
Patients
3470 patients (A=1784, B=1686) treated with pegylated interferon plus ribavirin under real life conditions were documented between 2000 and 2007 from 23 large outpatient clinics:
- Matched Pairs I: 2408 patients (A=1204; B=1204),
- Matched Pairs II: 1696 patients (A=848; B=848).
Patients with chronic hepatitis C (CHC): In order to evaluate only patients with chronic hepatitis C, 56 patients with acute hepatitis C (29 of group A and 27 of group B) were excluded from the analysis. Therefore, the following analysis is based on 3 data sets of patients with chronic hepatitis C:
- Intent-to-treat (ITT): 3414 CHC-patients (A=1755; B=1659),
- Matched Pairs I: 2378 CHC-patients (A=1189; B=1189),
- Matched Pairs II: 1672 CHC-patients (A=836; B=836).
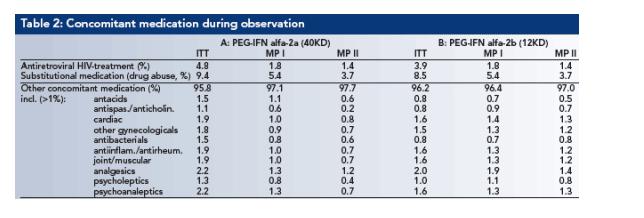
Demographic data for the 3 data sets are shown in Table 1. Concomitant treatments are shown in Table 2. Most of the patients were treatment naive (89.0%, ITT). 5.9% of the patients were relapsers, 4.9% non-reponders and 0.3%
were both relapsers and non-responders (³2 pretreatments).
Sources of infection were (ITT; multiple answers possible): i.v. drug abuse (35.7%), transfusion (18.0%), medical action (6.1%). The source of infection was unknown in 33.0% of the patients.
Early virological response (EVR)
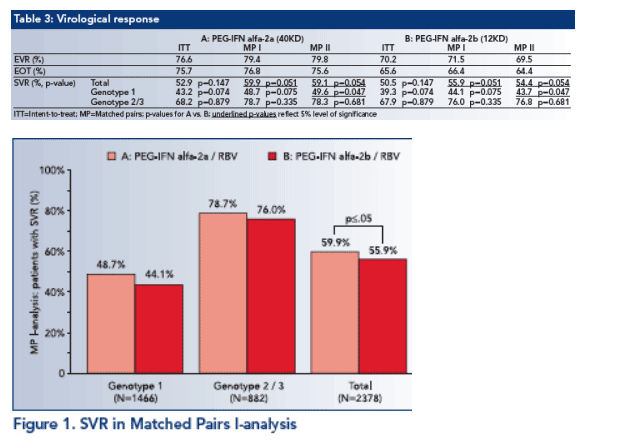
76.6% (A) vs. 70.2% (B) of the ITT-patients achieved Early virological responses at week 12 (³2-log10 drop in HCV RNA and/ or HCV RNA ≦50 IU/ml and/or HCV RNA qualitatively undetectable; see Table 3).
End of treatment response (EOT)
End of treatment response (EOT) was achieved in 75.7% (A) vs. 65.6% (B) of the ITT-patients (HCV RNA ≦50 IU/ml and/or HCV RNA qualitatively undetectable) (see Table 3).
Sustained virological response (SVR)
For group A-patients the SVR (HCV RNA ≦50 IU/ml and/ or HCV RNA undetectable after 24 weeks of follow-up) was 52.9% in ITT analysis. The respective SVR for the group B patients was 50.5% (see Table 3).
In the Matched pairs I-analysis the SVR was significantly lower in group B (55.9%) than in group A (59.9%) (p≦.05; see Fig. 1).
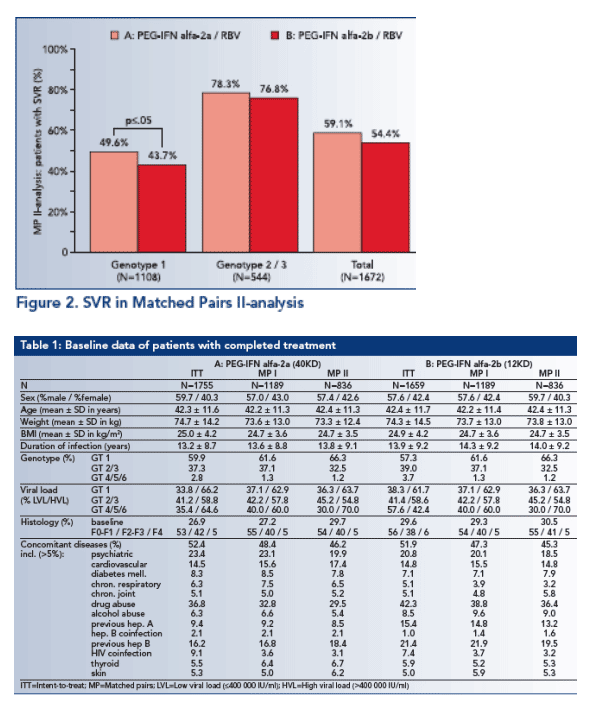
In the Matched pairs II-analysis the SVR for genotype 1-patients of group A (49.6%) was significantly lower (this must be a misprint because group A has higher SVR) than for group B-patients (43.7%; p≦.05; see Figure 2).
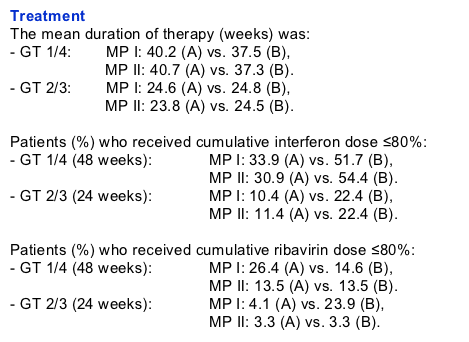
Treatment discontinuations (ITT)
21.8% of group A and 29.6% of group B discontinued therapy before end of treatment. Main reasons for withdrawal were (multiple answers possible):
- virological nonresponse (A: 58.2%; B: 65.2%),
- poor tolerability (A: 18.5%; B: 14.5%),
- patient request (A: 9.9%; B: 10.6%) and
- non-compliance (A: 8.9%; B: 4.1%).
CONCLUSIONS
For a valid comparison of peginterferon regimes it is important to match patient groups not only according to baseline factors but also according to ribavirin dose.
In our matched pairs analysis significantly more patients were cured with peginterferon alfa-2a (40KD) compared to peginterferon alfa-2b (12KD) in a real-life setting.
Further evaluations will have a closer view to possible reasons of our results concerning the different treatment outcomes of both peginterferons
|
| |
|
 |
 |
|
|