| |
Treatment of Chronic Hepatitis C Virus (HCV) Genotype 1 Patients with the NS3/4A Protease Inhibitor ITMN-191 Leads to Rapid Reductions in Plasma HCV RNA: Results of a Phase 1b Multiple Ascending Dose (MAD) Study
|
| |
| |
Reported by Jules Levin
AASLD Nov 4 2008 San Francisco, CA
N. Forestier1, D. Larrey2, D. Guyader3, P. Marcellin4, R. Rouzier5, A Patat6, B. Bradford7, S. Porter7, L. Blatt7, S. Zeuzem1
1J.W. Goethe Universitat, Frankfurt, Germany; 2CHU Montpellier, France; 3Pontchaillou Hospital, Rennes, France; 4Hopital Beaujon, Clichy, France; 5Centre CAP, Montpellier, France; 6Biotrial, Rennes, France; 7InterMune, Inc., Brisbane, CA
AUTHOR SUMMARY and CONCLUSIONS
ITMN-191 was safe and well tolerated when administered as monotherapy for 14 days to patients with chronic HCV genotype 1 infection
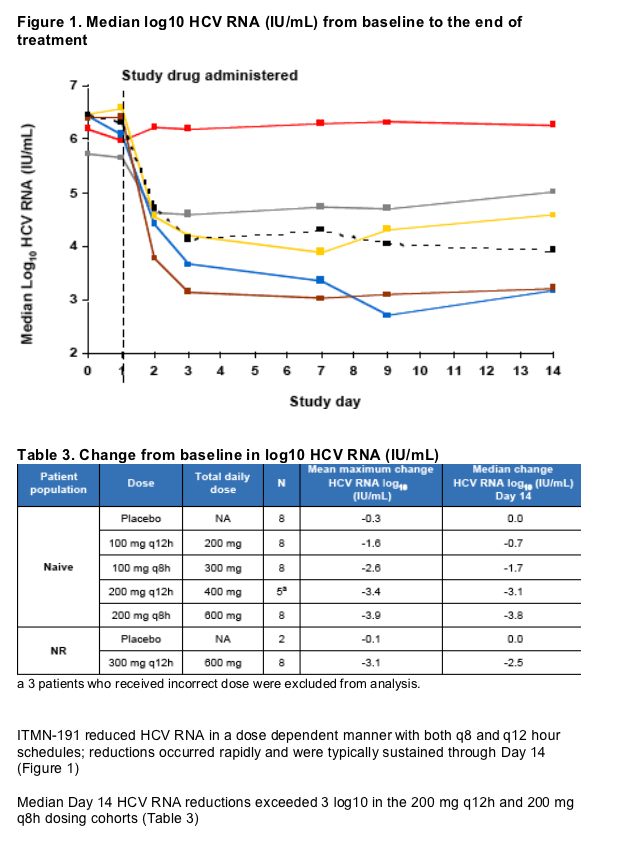
Treatment resulted in rapid and sustained reductions in HCV RNA with median reductions at Day 14 exceeding 3 log10 in both the 200 mg q8h and 200 mg q12h dosing cohorts (a median reduction at day 14 of 3.8 log10 in patients receiving 200 mg q8h). The robust antiviral activity and favorable safety observed following monotherapy with low doses of ITMN-191 support it's continued clinical development, including the initiation of studies designed to assess the safety and efficacy of ITMN-191 in triple combination regimens
Significant antiviral activity was seen in a single exploratory cohort of patients who had failed to respond to standard therapy. While the study design precludes meaningful comparisons with the treatment-naive cohorts, this observation will inform the design of subsequent dose ranging studies in this difficult-to-treat subpopulation
Extensive sequencing analysis is ongoing to assess the effect of viral variants on virologic response
Based on these findings, Phase 1b studies to assess the safety and efficacy of ITMN-191 in combination with either peginterferon a-2a plus ribavirin or oral STAT-C agents are currently underway
ABSTRACT
Background: ITMN-191 is an oral, highly potent, selective inhibitor of the HCV NS3/4A serine protease. Emerging evidence suggests combination regimens including direct inhibitors of viral enzymes will improve virologic response rates relative to the current standard of care. In order to identify doses for subsequent studies of ITMN-191-based combination regimens, we evaluated the safety, pharmacokinetics, and effects on plasma HCV RNA of multiple ascending doses of ITMN-191 in patients with chronic HCV infection.
Methods: In a double-blind, randomized, placebo-controlled study, 4 cohorts of treatment-naive HCV genotype 1 patients and one cohort of non-responders (NR) were randomized to receive ITMN-191 or matched placebo for 14 days. Cohorts consisted of 8 and 2 patients receiving ITMN-191 and placebo, respectively. Patients were sequestered in a Phase 1 unit for 16 days and completed standardized clinical and laboratory evaluations.
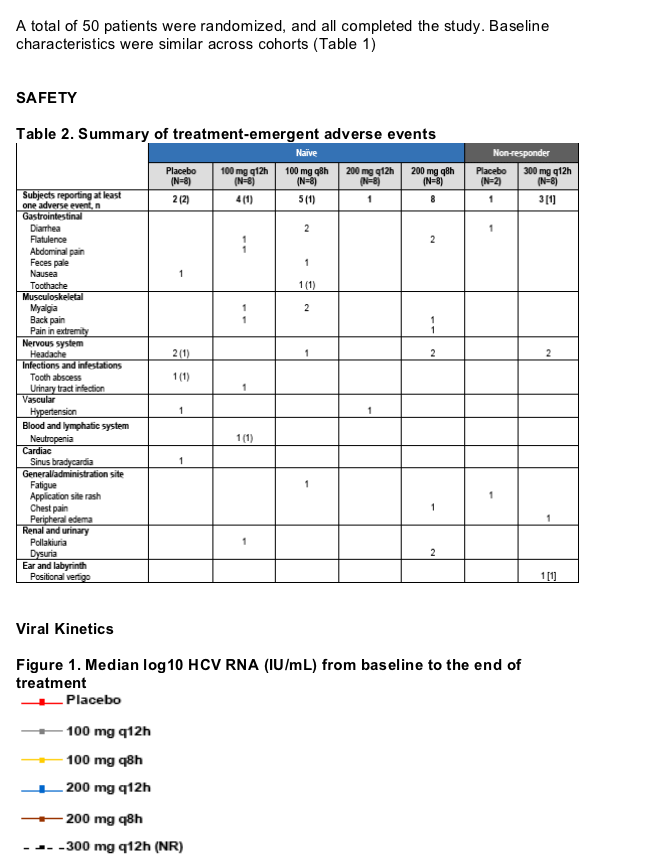
Results: Fifty patients were randomized and all completed the study. ITMN-191 was safe and well-tolerated. Adverse events were generally mild and transient and without association to treatment group or dose level. A single serious adverse event of benign paroxysmal positional vertigo was observed in a patient with a history of similar symptoms. The event was deemed unrelated to study drug and resolved rapidly without study drug discontinuation. ITMN-191 reduced HCV RNA in a dose dependent manner with both q8 and q12 hour schedules; reductions occurred rapidly and were typically sustained through day 14. Viral variants with reduced drug sensitivity were observed in the subset of patients that experienced virologic rebound but not in those who experienced a continual decline in HCV RNA.
Conclusion: ITMN-191 was safe and well tolerated. Treatment resulted in rapid and sustained reductions in HCV RNA, with a median reduction at day 14 of 3.8 log10 in patients receiving 200 mg q8h. Treatment response was lower in the NR cohort; this observation will inform regimen selection in future studies in this population. Based on these findings, a Phase 1b study to assess the safety and efficacy of ITMN-191 in combination with peginterferon é-2a plus ribavirin is currently underway.
BACKGROUND and AIMS
ITMN-191 is an oral, highly potent, selective inhibitor of the HCV NS3/4A serine protease. Emerging evidence suggests combination regimens including direct inhibitors of viral enzymes will improve virologic response rates relative to the current standard of care. In order to identify doses for subsequent studies of ITMN-191-based combination regimens, we evaluated the safety, pharmacokinetics (PK), and effects on plasma HCV RNA of multiple ascending oral doses of ITMN-191 in patients with chronic HCV infection.
METHODS
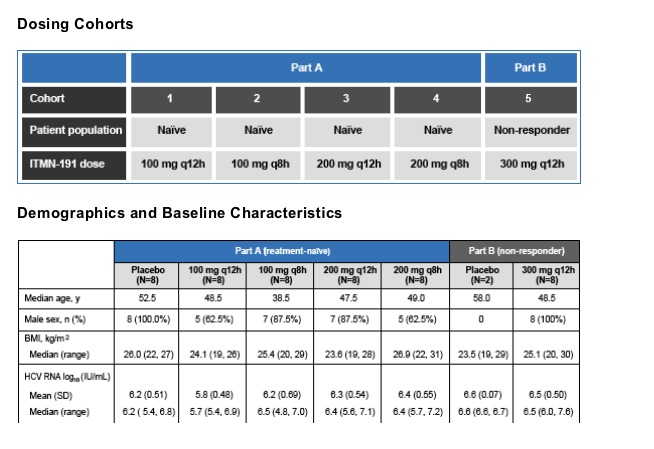
In a double-blind, randomized, placebo-controlled study, 4 cohorts of treatment-naive HCV genotype 1 patients and one cohort of nonresponders (NR) were randomized to receive ITMN-191 or matched placebo for 14 days
The study was comprised of two parts. Part A was a dose ranging study of multiple ascending doses of ITMN-191 in treatment-naive patients; in Part B, a single dose level was explored in a single cohort of non-responders. Non-responders were defined as patients who had failed to achieve a ≥
2 log10 reduction in HCV RNA at week 12 or undetectable HCV RNA at Week 24 or beyond during a standard course of therapy with pegylated interferon-a plus
ribavirin
Each cohort consisted of 8 and 2 patients receiving ITMN-191 and placebo, respectively. In Part A, 4 cohorts of treatment naive patients were treated with total daily doses ranging from 200-600 mg for 14 days; in Part B, a single cohort of nonresponders was treated with a total daily dose of 600 mg for 14 days
Patients were observed in a Phase 1 research facility from the night before the first dose until 48 h following the last dose of study drug (Day 16). Study drug was administered as an oral gelatin capsule 20-30 minutes after a standardized meal. Blood samples for PK and viral kinetic analyses were collected at baseline and periodically throughout the dosing period
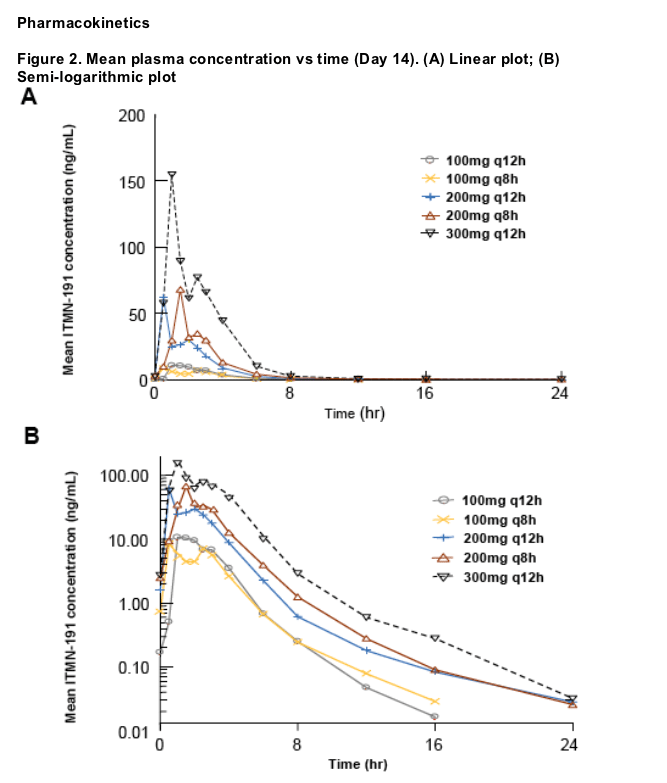
Oral administration of ITMN-191 resulted in robust antiviral activity at plasma concentrations substantially lower than those of other known NS3/4A protease inhibitors, suggesting that the high liver:plasma concentration ratios observed in preclinical species are also achieved in patients with chronic HCV infection
The relationship between dose and exposure was not uniformly proportional over the range of studied doses
|
|
| |
| |
|
|
|