| |
Greater Tenofovir-Associated Renal Function Decline with Protease Inhibitor-Based versus Nonnucleoside Reverse-Transcriptase Inhibitor-Based Therapy
|
| |
| |
"Treatment with TDF and PI/r was associated with greater declines in renal function over 48 weeks compared with TDF+NNRTI_based regimens."
The Journal of Infectious Diseases Jan 1, 2008;197:102-108
Miguel Goicoechea,1 Shanshan Liu,2 Brookie Best,1 Shelly Sun,1 Sonia Jain,1 Carol Kemper,3 Mallory Witt,4 Catherine Diamond,5
Richard Haubrich,1 Stan Louie,2 and the California Collaborative Treatment Group 578 Team
1University of California, San Diego, San Diego, 2University of Southern California, Los Angeles, 3Santa Clara Valley Medical Center, San Jose, 4LA Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, and 5University of California, Irvine, Irvine
Potential conflicts of interest: none reported.
Presented in part: 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, 25-28 February 2007 (abstract 835).
Financial support: Universitywide AIDS Research Program (grant CH05-SD-607-005); National Institute of Allergy and Infectious Diseases (grants K24 AI064086 and K23 AI066901); National Cancer Institute (grant 5P30 CA 14089-27); University of California, San Diego, Center for AIDS Research (grant 5P30 AI 36214); Health Resources and Services Administration (grant HAB 6 H4A-HA00016-02).
(See the editorial commentary by Szczech, on pages 7-8.)
ABSTRACT
Background. Plasma concentrations of tenofovir increase when the drug is coadministered with some ritonavir_boosted protease inhibitors (PI/r). We hypothesized that tenofovir disoproxil fumarate (TDF)-treated patients taking PI/r_based regimens would have a greater decline in renal function than patients receiving nonnucleoside reverse_transcriptase inhibitor (NNRTI)-based therapy.
Methods. We compared the estimated decline in renal function among 146 human immunodeficiency virus type 1 (HIV_1)-infected patients receiving a TDF+PI/r_ (n=51), TDF+NNRTI_ (n=29), or non-TDF_containing (n=66) regimen. Plasma tenofovir concentrations were measured at study week 2, and rates of creatinine clearance (CrCl) were estimated using the Cockcroft_Gault (C_G) and Modification of Diet in Renal Disease (MDRD) equations. Mixed_effects models were used to analyze regimen type and tenofovir concentration as predictors of change in CrCl from baseline to weeks 24 and 48.
Results. Decreases in C_G estimates of CrCl were not significantly different among the 3 groups during the first 24 weeks of therapy. However, in adjusted analyses, patients receiving TDF+PI/r had a greater rate of decline in CrCl than did the TDF+NNRTI group (for C_G, _13.9 vs. _6.2 mL/min/year [p=.03]; for MDRD, _14.7 vs. _4.5 mL/min/1.73 m2/year [p=.02]). Among TDF_treated patients, tenofovir plasma concentration was not associated with CrCl over time.
Conclusions. Treatment with TDF and PI/r was associated with greater declines in renal function over 48 weeks compared with TDF+NNRTI_based regimens.
Tenofovir disoproxil fumarate (TDF) is an oral prodrug of tenofovir and, like cidofovir and adefovir, is an acyclic nucleoside phosphonate. Tenofovir is eliminated by renal clearance, largely by glomerular filtration, with 20%-30% being actively transported into renal proximal tubule cells by organic anion transporter (OAT)-1 [1]. Once inside the cell, tenofovir is excreted into the urine by multidrug resistance protein (MRP)-2 [2, 3] and MRP_4 [4]. Controlled clinical studies of TDF used in efavirenz_based regimens have demonstrated low rates of renal toxicity [5, 6]. However, conflicting data regarding an increased incidence of TDF_associated nephrotoxicity in combination with ritonavir_boosted protease inhibitor (PI/r)-based therapy have been reported [7-9].
Some PI/r_based therapies can increase plasma exposure of tenofovir by 20%-30% [10, 11]. In animal studies, high doses of TDF for prolonged periods led to a Fanconi_like syndrome with reduced renal tenofovir clearance [12]. At present, the mechanism for increased tenofovir exposure during PI/r coadministration is unclear. Ray et al. [13] observed in vitro that TDF is a substrate for P glycoprotein and that PI/r inhibition of this transporter in enterocytes may increase absorption and systemic exposure of tenofovir. Alternatively, in vivo studies in HIV_infected patients have demonstrated reduced tenofovir renal clearance among patients receiving a PI/r than among non-PI_treated patients [14, 15], suggesting that impaired renal excretion leads to increased tenofovir plasma concentrations.
The objective of the present study was to investigate the relationship between PI/r coadministration with TDF and changes in estimated renal function. Comparator groups included HIV_infected patients receiving TDF and nonnucleoside reverse_transcriptase inhibitor (NNRTI)- and non-TDF_containing treatment regimens. In addition, steady_state (week 2) tenofovir plasma exposures were determined to evaluate the association between tenofovir concentration and longitudinal changes in renal function.
DISCUSSION
Renal impairment is a common problem among HIV_infected patients, and the uncertainty surrounding TDF's potential for renal toxicity is a current therapeutic dilemma. Of the 146 subjects in the present longitudinal study, those receiving concurrent TDF+PI/r treatment had greater reductions in CrCl than did patients taking TDF+NNRTI_ or non-TDF_based regimens. Given that some PI/r_based therapies increase systemic tenofovir exposure [10, 11], understanding this mechanism and whether it is due to increased intestinal absorption or decreased renal excretion will have disparate clinical implications. If the cause of the pharmacokinetic drug interaction is within the renal cell itself, then a reduction in the dose of TDF would not necessarily decrease the risk of nephrotoxicity with PI/r coadministration.
Ritonavir is a potent inhibitor of MRP_2 [22]. Although in vitro studies do not agree on the role played by MRP_2 in the cellular transport of tenofovir [2, 4], experiments in animal models using wild_type and MRP_2-deficient (GY/TR_) rats confirm that tenofovir is transported by MRP_2 [3]. Furthermore, recent clinical data demonstrate that genetic polymorphisms in MRP_2 promoter and/or coding regions are associated with differences in urinary tenofovir excretion [23] and the probability of developing TDF_associated renal proximal tubulopathy [24] in HIV_infected patients.
Impaired renal tenofovir excretion by PI/r_based therapies would explain 2 observations. First, increased rates of renal insufficiency have not been observed in large randomized trials of TDF in combination with efavirenz [5, 6]. NNRTIs do not inhibit renal transporters [25]. Coadministration of this drug class would not impair renal clearance, with consequent higher intracellular tenofovir accumulation and cytotoxicity. Therefore, the risk of renal impairment with this regimen combination should be low. Second, we found no association between steady_state tenofovir plasma exposure and change in CrCl over time-even after adjusting for baseline renal function. It is possible that intracellular tenofovir and/or its phosphorylated anabolites are linked more strongly with renal cell cytotoxicity and that plasma tenofovir levels may correlate poorly with these concentrations.
Differences in outcome between our study and other observational studies of TDF_ and PI/r_treated patients can be attributed to differences in cohort selection. The HIV Outpatient Study (HOPS) assessed the effect of TDF in combination with lopinavir_ritonavir or atazanavir_ritonavir (n=99) versus TDF+NNRTI or other PI_based regimens (n=210) on change in renal function [7]. After 12 months of follow_up, the groups had no significant differences with respect to CrCl decline (_5.1 vs. _2.8 mL/min [p=.51]). The smaller observed change in renal function among these PI/r_treated patients may have been the result of differences in patient characteristics. The HOPS cohort included only antiretroviral_naive patients with less_advanced HIV disease (median CD4+ T cell count, 352-427 cells/mm3), compared with those in the present study (median CD4+ T cell count, 184-220 cells/mm3). Moreover, the comparator group contained both TDF_ and other PI+TDF_treated patients, which may have decreased renal function in this study arm, reducing the size of the effect and the power to detect a difference between treatment groups. Indeed, Winston et al. [9] did observe significant changes in renal function among antiretroviral_naive patients with high CD4+ T cell counts (mean CD4+ T cell count, 381-461 cells/mm3) receiving either a TDF_ n=290 or non-TDF_ (n=618) containing regimen. In the present study, TDF_treated patients (70% with concurrent PI/r) did have significantly greater time_weighted declines in CrCl than did non-TDF_treated patients (_5.6 mL/min vs. 1.26 mL/min [p=.01]).
Patients with advanced disease are at increased risk for chronic kidney disease [26] and may be more susceptible to tenofovir_associated renal toxicity. In the Johns Hopkins clinical cohort, composed of individuals with low baseline CD4+ T cell counts (median CD4+ T cell count, 214-220 cells/mm3), renal function decline between individuals receiving TDF (n=344) versus other nucleoside_based (n=314) regimens was compared [8]. Although only 36% of the TDF_treated patients received concurrent PI/r therapy, this group had significantly greater declines in renal function than did non-TDF_treated patients (_13.3 vs. _7.5 mL/min [p=.005]). Of note, the magnitudes of CrCl declines were similar to the changes observed in our study for TDF+PI/r_ and non-TDF_treated patients (_13.9 mL/min and _6.0 mL/min, respectively), which may reflect baseline similarities between the study cohorts.
This retrospective analysis had several limitations, most notably that HIV regimens were not randomized at baseline and that significant differences were seen in the proportion of treatment_experienced patients between groups. Patients receiving TDF and NNRTIs were more often treatment naive than were TDF+PI/r_treated individuals (38% vs. 10%). Potentially, treatment_experienced patients may be more predisposed to renal adverse events. However, all groups had similar, normal_range CrCl rates at baseline, and adjusted analyses between TDF+PI/r_ and non-TDF_treated patients demonstrated the independent effect of a TDF+PI/r regimen even after adjusting for treatment experience. In addition, tenofovir plasma exposure was measured only after 2 weeks of therapy. This may have been too early to observe the full effects of PI inhibition of MRP_2. Potentially, serial measurements over longer periods may capture interpatient differences in plasma tenofovir concentrations due to MRP_2 inhibition and associate better with change in renal function over time. Furthermore, data on the proportion of subjects with diabetes mellitus, hypertension, proteinuria, and concurrent nephrotoxic medications were not available. Knowing that these potential confounders were not more common in one group than another would have been reassuring.
Differences in HIV disease outcome among treatment groups could have an independent effect on kidney function. The C_G and MDRD equations include variables such as age, sex, race, body weight, blood urea nitrogen level, albumin level, and serum creatinine level as surrogates for muscle mass. Body weight increased to a greater extent in the TDF+NNRTI and non_TDF groups than in the TDF+PI/r group. However, this difference in weight gain represents a conservative bias and provides evidence that the observed increases in estimated CrCl in the TDF+PI/r group were not due to a relative increase in muscle mass. In addition, low CD4+ T cell counts and high HIV_1 RNA loads are considered to be risk factors for chronic kidney disease [26]. Although viral suppression was greater in the TDF+NNRTI group (probably because of the higher proportion of treatment_naive patients in this group), the difference in viral load compared with that in the TDF+PI/r group was small (change in HIV_1 RNA load, 1259 copies/mL) and was likely not clinically relevant, given that CD4+ T cell recovery was similar among the 3 treatment groups.
In conclusion, the present study demonstrated that patients receiving TDF in combination with PI/r_based regimens had greater declines in renal function than did TDF+NNRTI_ or non-TDF_treated individuals. Although the magnitude of CrCl decline was relatively small, if it were to continue throughout the life of a patient, it would have serious clinical implications. In our cohort, the only risk factor for significant 1_year renal function decline (>15% decrease from baseline at week 48) among patients initiating TDF_based therapy was concurrent use of a PI/r_containing regimen. We postulate that the mechanism is a drug_drug interaction at the level of renal proximal tubule cell, whereby PI/r_based therapies inhibit tenofovir efflux, resulting in greater intracellular accumulation. Moreover, genetic polymorphisms in renal tubular transporters may modify this risk [27-29]. Future investigations are needed to identify individuals with polymorphisms for high OAT_1 expression and/or low_functioning MRP_2/4 transporters, because these patients may be at increased risk for renal toxicity.
METHODS
Study population. Subjects included in this analysis were identified from California Collaborative Treatment Group (CCTG) 578, a prospective, randomized clinical trial of therapeutic drug monitoring of antiretroviral therapy [16]. In this study, patients were recruited from 5 HIV outpatient clinics in California on the basis of the need to initiate a new HIV regimen and to improve medication adherence behaviors, as determined by the health care provider and/or the patient's self_description. Subjects were antiretroviral naive or experienced and had baseline detectable plasma HIV_1 RNA loads regardless of whether they were not currently receiving therapy or experiencing treatment failure with their current regimen. All patients initiated a new PI/r_ or NNRTI_based regimen at study entry. Subjects were eligible for the present analysis if they had serum creatinine values available at baseline and at week 48 of therapy. Because the renal effects of tenofovir were hypothesized to be dependent on exposure, TDF_treated subjects were included if they received continuous TDF treatment for at least 40 weeks; otherwise, all other subjects receiving a PI/r_ or NNTRI_based therapy and not receiving TDF were analyzed. The duration of antiretroviral therapy among the 3 treatment groups was comparable. Appropriate written informed consent was obtained from all study participants.
Measurements. Rates of creatinine clearance (CrCl) were estimated using the Cockcroft_Gault (C_G) equation [17] and the unabbreviated Modification of Diet in Renal Disease (MDRD) equation (equation 7) [18]. The subject's ideal body weight was used in the C_G estimations. In CCTG 578, serum creatinine levels were routinely monitored (baseline and weeks 2, 6, 12, 24, 32, 40, and 48) and measured at a central laboratory (Quest Diagnostics). Estimates of renal function were based on creatinine values obtained at weeks 24 and 48; however, if serum creatinine laboratory values were not available from these time points, values at weeks 32 and 40 were used, respectively. Tenofovir concentrations were measured at the Norris Cancer Center PharmacoAnalytical Laboratory in stored plasma samples collected before and 2 and 4 h after a witnessed medication dose at study week 2, by a validated liquid_chromatography mass_spectroscopy method. The calibration curve range for tenofovir in human plasma was 10-1000 ng/mL, with a lower limit of quantification of 10 ng/mL. The assay was linear over this range (r2>0.99) and demonstrated excellent interday accuracy and precision. Individual tenofovir pharmacokinetic parameters, including tenofovir maximum concentration (Cmax), minimum concentration (Cmin), and oral clearance (CL/F), were determined for each subject by use of the POSTHOC subroutine in the computer program NONMEM (version V.1; Globomax). A 2_compartmental model and initial population pharmacokinetic parameter estimates from a published population pharmacokinetic study were used for the base population model without covariates [15].
Statistics. Linear mixed_effects models [19, 20] were used to study the relationship between HIV treatment regimen and CrCl over multiple time points (at baseline, week 24, and week 48). The primary fixed_effects covariates in the models were regimen type (TDF+PI/r based vs. TDF+NNRTI based or non_TDF based), time, and the interaction term "regimen type _ time." The intercept term was allowed to vary across individuals and was treated as the only random effect in the model. The interaction term in the model represented the difference in the slopes of change in CrCl over time between the different treatment groups. Other potential confounders-such as age, race (white vs. nonwhite), sex, baseline CD4+ T cell count, baseline HIV_1 RNA load, and treatment history (experienced and receiving therapy, experienced and not receiving therapy, and naive)-were included in the model on the basis of clinical considerations. The influence of these covariates on CrCl over time was studied both univariately and in the multivariate mixed_effects model. Sensitivity analysis and model diagnostics were performed under different mixed_effects modeling assumptions, to validate the results.
We also used mixed_effects models to study the relationship between tenofovir plasma exposure at week 2 (Cmin, Cmax, or CL/F) and CrCl over time. In these models, plasma exposure, time, and the interaction term "plasma exposure _ time" were included as the main fixed effects, and intercept was the only random effect. Logistic regression models evaluated baseline factors as predictors of a significant decline in CrCl (defined as a >15% decrease from baseline) among TDF_treated patients. Student's t tests were used for comparisons of change in CrCl between treatment groups at week 24 and 48. Statistical analysis was performed using R (version 2.4.1).
RESULTS
Patient characteristics. One_hundred ninety_nine patients participated in the parent study, of whom 48 who did not initiate or discontinued TDF before week 48, 3 who received TDF without a PI/r or an NNRTI, and 2 who received TDF with a baseline CrCl rate <50 mL/min were excluded from this analysis. Of the remaining 146 patients, 51 received a TDF+PI/r_, 29 received a TDF+NNRTI_, and 66 received a non-TDF_containing HIV treatment regimen (table 1). Of note, 4 patients treated with TDF and both an NNRTI and PI/r were assigned to the PI/r group. No significant differences were noted between treatment groups with respect to age, sex, race, or baseline CD4+ T cell count. Patients receiving TDF+NNRTI were more often treatment naive and had higher baseline HIV_1 RNA loads than did patients receiving a PI/r. The mean baseline renal function was within the normal range and was similar between treatment groups by either estimate of CrCl (C_G range, 102-111 mL/min [p=.17]; MDRD range, 106-110 mL/min/1.73 m2 [p=.79]). Most patients in the TDF+PI/r group received lopinavir_ritonavir (75%), whereas efavirenz (79%) was most commonly used in the TDF+NNRTI group. In the non_TDF group, approximately half received a PI/r, and the rest received an NNRTI.
Change in estimated renal function (C_G). In univariate analysis, decreases in C_G estimates of CrCl were not significantly different among the 3 groups during the first 24 weeks of therapy (mean [SE], _7.75 [2.2] for TDF+PI, _4.46 [3.1] for TDF+NNRTI, and _1.14 [2.8] mL/min for non_TDF) (figure 1). After week 48, the TDF+NNRTI and non_TDF groups continued to have similar changes in renal function from baseline (mean [SE], _6.24 [2.6] and _6.02 [3.5] mL/min, respectively [p=.96]). However, TDF+PI/r_treated patients had greater week 48 declines in CrCl than did patients receiving a TDF+NNRTI_based regimen (mean [SE], _13.9 [2.4] and _6.24 [2.6] mL/min, respectively [p=.04]). In addition, increasing age (p=.001) and female sex (p<.001) were associated with lower baseline CrCl, whereas race, baseline CD4+ T cell count, baseline HIV_1 RNA load, and treatment history were not.
Figure 1. Regimen type and change in renal function. The solid line with white circles represents individuals receiving a non-tenofovir disoproxil fumarate (TDF)-based regimen, the dashed line with black circles represents individuals receiving a TDF plus ritonavir_boosted protease_inhibitor (PI/r)-based regimen, and the solid line with white triangles represents individuals receiving a TDF plus nonnucleoside reverse_transcriptase inhibitor (NNRTI)-based regimen. Student's t tests were used for comparisons between regimen types. Errors bars represent SEs. *Comparisons between the TDF+NNRTI group and the TDF+PI/r or non_TDF group. Comparison between the TDF+NNRTI group and the TDF+PI/r group. NS, nonsignificant.
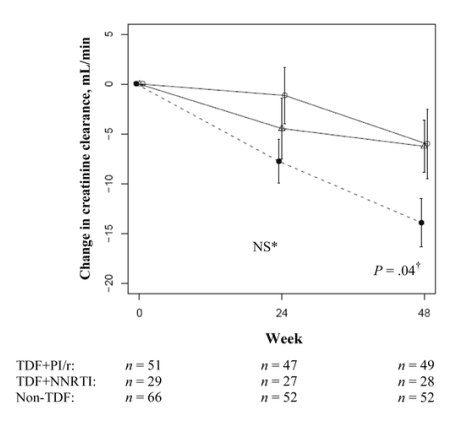
In mixed_effects analysis, patients receiving TDF+PI/r had an increased rate of decline in CrCl compared with the TDF+NNRTI group over 48 weeks (mean [SE], 7.66 [3.6] mL/min/year []) (table 2). In addition, baseline CrCl decreased by 1.15 mL/min for every 1 year increase in patient age (p<.001), and women had lower baseline CrCl than men (22.3 mL/min lower []). Compared with patients treated with non-TDF_containing regimens, the TDF+PI/r group also had a greater rate of renal function decline after 48 weeks of therapy (mean [SE], 7.88 [3.73] mL/min/year [p=.04]). In this model, age and sex continued to be significantly associated with baseline renal function; in addition, individuals with higher baseline CD4+ T cell counts (p=.08) and HIV_1 RNA loads (p=.09) trended toward higher initial CrCl.
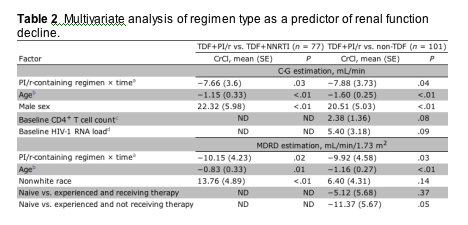
Note. Mixed_effects models were used for these analyses. The outcome variable was rate of creatinine clearance (CrCl) at baseline, week 24, and week 48, estimated by the Cockcroft_Gault (C_G) or Modification of Diet in Renal Disease (MDRD) equation, for each model. Covariates were included in the multivariate model if significant at a=0.25 in univariate analysis. ND, not done; NNRTI, nonnucleoside reverse_transcriptase inhibitor; PI/r, ritonavir_boosted protease inhibitor; TDF, tenofovir disoproxil fumarate.
aRelative difference in change in CrCl over 48 weeks between a
TDF+PI/r_containing regimen and a TDF+NNRTI_ or non-TDF_containing regimen.
bChange in baseline CrCl per 1_year increase.
cChange in baseline CrCl per 100 CD4+ T cells/mm3 increase.
dChange in baseline CrCl per 1.0 HIV RNA log10 copies/mL increase.
Change in estimated renal function (MDRD). Repeat mixed_effects analysis using the MDRD equation yielded results similar to those of the C_G estimation (table 2). In this analysis, the TDF+PI/r group had a greater rate of renal function decline than did both the TDF+NNRTI (mean [SE], 10.15 [4.23] mL/min/1.73 m2/year [p=.02]) and non_TDF groups (mean [SE], 9.92 [4.58] mL/min/1.73 m2/year [p=.03]). Increasing age was also associated with lower initial estimates of CrCl (1 mL/min/1.73 m2 decline per year). Also, in the model including non-TDF_treated patients, treatment_experienced patients not receiving therapy at baseline had significantly lower initial CrCl (mean [SE], _11.37 [5.67] mL/min/1.73 m2 [p=.05]) than did antiretroviral_naive patients.
Plasma concentrations of tenofovir. We sought to determine a possible relationship between steady_state (week 2) tenofovir plasma concentrations and subsequent changes in renal function. Patients receiving a PI/r had tenofovir plasma concentrations and oral clearance rates similar to those of NNRTI_treated individuals (Cmax, 255 vs. 225 ng/mL [p=.34]; Cmin, 76 vs. 63 ng/mL [p=.18]; CL/F, 96 vs. 108 L/h [p=.36]). In mixed_effects models, after adjustment for age, none of these pharmacokinetic parameters were associated with CrCl over time. Furthermore, no association was found between the week 2 population_predicted individual plasma concentrations of PI/rs or NNRTIs (Cmin or Cmax) and CrCl over time (data not shown).
Effect of HIV treatment outcomes on renal function. All 3 treatment groups had persistent, low_level viremia after 48 weeks of therapy. Patients receiving TDF+PI/r_based regimens had significantly greater HIV_1 RNA loads than did TDF+NNRTI_treated patients (mean [SD], 1622 [25] vs. 363 [10] copies/mL [p=.03]) but had levels similar to those in non-TDF_treated subjects (mean [SD], 813 [20] copies/mL [p=.28]). Importantly, the proportion of subjects with complete viral suppression (HIV_1 RNA load <50 copies/mL) was not significantly different among treatment groups. Furthermore, CD4+ T cell recovery was similar among all 3 treatment groups, and there were no significant differences in mean CD4+ T cell counts at week 48 (mean range, 323-362 cells/mm3). In separate mixed_effects models, longitudinal changes in CD4+ T cell counts and HIV_1 RNA loads were not associated with CrCl and did not change the effect of treatment group on renal function decline (data not shown).
Increases in body weight may increase serum creatinine levels and decrease estimated kidney function [21]. Over 48 weeks of follow_up, TDF+NNRTI_treated patients had the greatest increase in body weight (mean, 9.1_kg gain), followed by patients receiving non-TDF_ (mean, 7.3_kg gain) and TDF+PI/r_based (mean, 0.8_kg gain) regimens. However, in adjusted analysis, these changes in body weight were not associated with the observed differences in CrCl among the treatment groups (data not shown).
Predictors of significant renal function decline. Thirty_four percent (26/77) of patients receiving TDF developed significant renal function decline (defined as a >15% decrease in C_G-estimated CrCl) after 48 weeks of therapy. There was no statistically significant association between age, sex, race (white vs. nonwhite), baseline CrCl, or week 2 tenofovir plasma exposures and 1_year renal function decline. However, in adjusted analysis of baseline HIV_1 loads, the odds of developing significant renal function decline among TDF_treated patients was 3.7 times higher for subjects receiving concomitant PI/r versus those receiving NNRTI_based therapy (p=.04).
|
|
| |
| |
|
|
|