| |
Lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and two nucleosides for maintenance therapy of HIV
|
| |
| |
AIDS:Volume 22(2)11 January 2008p F1-F9
Pulido, Federicoa,*; Arribas, Jose Rb,*; Delgado, Rafaela; Cabrero, Estherc; Gonzalez-Garcia, Juanb; Perez-Elias, Maria Jd; Arranz, Albertoe; Portilla, Joaquinf; Pasquau, Juang; Iribarren, Jose Ah; Rubio, Rafaela; Norton, Michaeli; for the OK04 Study Group
From the aUnidad HIV and Laboratorio de Microbiologia Molecular, Hospital 12 de Octubre, Universidad Complutense de Madrid, Madrid, Spain
bServicio de Medicina Interna, Hospital La Paz, Universidad Autonoma de Madrid, Madrid, Spain
cAbbott Laboratories, Madrid, Spain
dHospital Ramon y Cajal, Madrid, Spain
eHospital Principe de Asturias, Alcala de Henares, Spain
fHospital General de Alicante, Alicante, Spain
gHospital Virgen de las Nieves, Granada, Spain
hHospital Donostia, San Sebastian, Spain
iAbbott Laboratories, Abbott Park, Ill, USA.
*F. Pulido and J.R. Arribas contributed equally to this study.
Abstract
Background: Prior attempts to reduce the number of drugs needed to maintain viral suppression in patients with suppressed HIV replication while receiving three antiretroviral drugs have been unsuccessful.
Methods: In 205 patients with suppressed HIV replication on lopinavir-ritonavir and two nucleosides, this randomized, open-label, non-inferiority clinical trial compared the strategies of continuation of triple therapy versus lopinavir-ritonavir monotherapy followed by reinduction with two nucleosides if virological rebound occurred without genotypic resistance to lopinavir-ritonavir. The primary endpoint was proportion of patients without therapeutic failure, defined as confirmed HIV RNA higher than 500 copies/mL (with exclusion of patients receiving monotherapy who resuppressed to < 50 copies/mL after resuming baseline nucleosides), or loss to follow-up, or change of randomized therapy other than reinduction.
Results: At week 48, the percentage of patients without therapeutic failure was 94% in the monotherapy group versus 90% in the triple therapy group (difference,-4%; upper limit of 95% confidence interval for difference, 3.4%). The percentage of patients with HIV RNA 50 copies/mL at 48 weeks by intention-to-treat, missing data or reinductions considered as failures, were 85% in the monotherapy group versus 90% in the triple therapy group (P = 0.31; 95% upper limit of 95% confidence interval for difference, 14%).
Conclusion: In this trial, 48 weeks of lopinavir-ritonavir monotherapy with reintroduction of nucleosides as needed was non-inferior to continuation of two nucleosides and lopinavir-ritonavir in patients with prior stable suppression. However, episodes of low level viremia were more common in patients receiving monotherapy. (ClinicalTrials. gov number, NCT00114933).
Introduction
A combination of three antiretrovirals is globally recommended for the treatment of HIV disease [1-3]. Three drugs are typically combined from two different classes; two nucleoside reverse transcriptase inhibitors plus one protease inhibitor or one non-nucleoside reverse transcriptase inhibitor. For the adherent patient, taking three drugs from two antiretroviral classes translates into a substantial and durable suppression of HIV replication. The successful viral control achieved by these combinations is mainly due to the high genetic barrier to resistance that is difficult for a population of wild-type HIV to escape from [4].
Despite the success of triple drug regimens, the idea or treating HIV with fewer than three drugs continues to be very attractive, due mainly to issues of cost, convenience and toxicity. Unfortunately, prior attempts at decreasing the number of drugs needed to maintain HIV viral suppression have been unsuccessful [5-7].
Lopinavir-ritonavir is an appropriate candidate for single drug therapy of HIV infection due to its high potency [8], its high genetic barrier to resistance [9] and its ability to reduce HIV replication in the cerebrospinal fluid [10]. We and others have preliminarily tested the hypothesis that lopinavir-ritonavir monotherapy might be sufficient to control HIV replication [11,12]. In a pilot trial [12] we showed that 17 of 21 (81%) patients who simplified from triple therapy to lopinavir-ritonavir monotherapy maintained HIV viral suppression after 72 weeks. None of the patients who failed lopinavir-ritonavir monotherapy in this pilot study developed mutations in the HIV protease gene. Importantly in three patients who failed lopinavir-ritonavir monotherapy, virological suppression could be regained by restarting prior nucleosides. As a follow up to the small pilot trial we conducted a larger clinical trial comparing lopinavir-ritonavir monotherapy versus lopinavir-ritonavir and two nucleosides for maintenance of HIV viral suppression.
Patients and methods
Study design
This randomized, open-label, investigator-initiated, non-inferiority, multicentre clinical trial was carried out in 28 sites in Spain. Patients were at least 18 years old, had no previous history of suspected or confirmed virological failure while receiving a protease inhibitor, were receiving two nucleoside reverse transcriptase inhibitors (or one nucleoside plus tenofovir DF) and lopinavir-ritonavir soft gel capsule (400/100 mg b.i.d.) for at least 4 weeks and had < 50 copies of HIV RNA/mL for at least the prior 6 months. Patients with a single transitory episode of detectable viral load ('blip', defined as an HIV-RNA viral load >50 copies/mL preceded and followed by one HIV-RNA viral load < 50 copies/mL without changes in antireteroviral treatment) during the prior 6 months could also been included. Exclusion criteria were pregnancy, presence of serum hepatitis B surface antigen in patients treated with lamivudine, emtricitabine or tenofovir DF, need for treatment with agents known to have potential major interactions with lopinavir-ritonavir and major psychiatric disease as assessed by the investigator. The study was approved by the Regional Ethics Committee for Clinical Research of the Community of Madrid, the local ethics committees for clinical research at each site and by the Spanish Agency for Medicines and Healthcare Products. All patients gave dated and written informed consent.
Patients were randomly assigned in a 1: 1 ratio to stop or continue the two nucleosides (or one nucleoside plus tenofovir DF). Randomization was centralized and computer generated (Clin Stat v.08.05.96. Department of Public Health Sciences, St George's Hospital Medical School). Randomization was stratified by CD4 cell count (< or ≥ 200 cells/μL) and months with HIV RNA < 50 copies/mL prior to randomization (< or ≥ 9 months).
Patients were assessed at baseline, week 4, week 12, and every 12 weeks thereafter until week 48. At study visits, clinical data was collected and labs were drawn after an overnight fast. Laboratory analyses included CD4 cell count, measurement of plasma HIV-1 RNA, haematology, plasma chemistry profiles, and a fasting lipid panel.
Plasma HIV-1 RNA and RNA HIV-1 genotyping were performed at the Laboratory of Molecular Microbiology at the Hospital 12 de Octubre in Madrid. All other laboratory determinations were performed locally at each site.
Adherence was assessed using a validated, 6-item, adherence questionnaire (GEEMA) [13]. Patients were classified as non-adherent when there was a positive response to any of the qualitative questions included. Responses to two of the questions in the questionnaire (a) 'Thinking about the last week. How often have you not taken your medicine?' and (b) 'Since the last visit how many days have you not taken any medicine at all?') were also independently quantified and analysed, as they had been associated with the risk of virological rebound in a previous pilot trial [12].
Virologic analysis
HIV-1 plasma viral load was performed by automatized RNA extraction in an Cobas AmpliPrep instrument followed by quantification using the Cobas TaqMan HIV-1 in a TaqMan 48 analyzer (Roche Molecular Systems, Inc., Branchburg, New Jersey, USA).
Any sample with detectable plasma HIV RNA (> 50 copies/mL) was repeated every 2 weeks during the following month, and then monthly until HIV viral load became undetectable again or until viral load increased > 500 copies/mL in two different samples. Viral genotyping was performed in all samples with viral load > 500 copies/mL. Antiretroviral susceptibility studies were performed in circulating virions by sequencing the protease (PR) and reverse transcriptase (RT) (amino acids 1-335) genes using the ViroSeq HIV-1 Genotyping system V2.0 (Celera Diagnostics, Alameda, California, USA). In the present analysis, lopinavir-ritonavir associated resistance mutations are defined according to the list published by the International AIDS Society-USA in 2006 [14]. Phenotypic testing (in selected samples) for antiretroviral resistance was performed by Antivirogram (Virco BVBA, Mechelen, Belgium). Results of phenotypic testing were not available to the investigators during the trial.
Endpoints
The primary objective was to assess the non-inferiority of the strategy of simplification to lopinavir-ritonavir monotherapy versus continuation of triple therapy. Subjects were permitted to restart nucleosides if monotherapy did not maintain virologic suppression as long as the virus remained without genotypic resistance to lopinavir-ritonavir [14].
The primary outcome measure was the proportion of patients without therapeutic failure at 48 weeks. Therapeutic failure was defined as any of the following: (i) two consecutive measurements of HIV RNA > 500 copies/mL separated by at least 2 weeks. A cut-off level of 500 copies/mL was chosen because it is probably a truer measure of virologic failure than a lower cut-off. Patients randomized to lopinavir-ritonavir monotherapy who failed by this definition were not considered therapeutic failures if at the time of failure there was no evidence of lopinavir-ritonavir genotypic resistance, were reinduced with two nucleosides and were suppressed to < 50 copies/mL of HIV RNA at 48 weeks; (ii) change of randomized therapy for reasons different from re-induction in the monotherapy group; (iii) treatment discontinuation; (iv) loss to follow-up; (v) for patients re-induced in the monotherapy group: decrease in HIV RNA < 1 log10 4 weeks after reinduction or failure to reach HIV RNA < 50 copies/mL 16 weeks after reinduction.
Secondary efficacy outcomes included the proportion of patients with virological failure (HIV RNA > 50 or > 500 copies/mL, according to the analysis) through week 48. For these secondary outcomes we used the FDA algorithm for time to loss of virologic response [15]. This algorithm requires consecutive confirmation of a value before assigning failure. Missing data, early termination of participation in the study, or re-induction with nucleosides in the monotherapy group were considered to be failures in these analyses. Additional secondary endpoints were the development of HIV resistance and changes in the CD4 cell count.
Comparisons between the two treatment groups were also made with respect to the frequency and severity of treatment-related adverse events, the incidence of laboratory abnormalities, and changes from baseline in laboratory values.
Statistical analysis
With 100 patients per group, the study has 80% power to show the non-inferiority of the monotherapy group, assuming 10% therapeutic failure rate at 48 weeks in both groups. We based this assumption on the results of our initial monotherapy pilot trial [12]. Per protocol, non-inferiority would be demonstrated if the upper limit of the 95% confidence interval (CI) of the difference in percentage of patients without therapeutic failure (triple therapy - monotherapy) was < 12%.
All randomized patients who received at least one dose of their assigned therapy were included in the analyses and they were followed for the entire 48 weeks regardless of whether they prematurely discontinued or switched their randomized therapy.
Treatment groups were compared using the Fisher exact test and ƒÔ2 test for categorical variables and the Mann-Whitney U test for continuous variables. Time-to-event analyses were performed using Kaplan-Meier survival curves and the log rank test. All reported P values are two-sided and were not adjusted for multiple testing.
Results
Between December 2004 and July 2005, 205 patients receiving lopinavir-ritonavir and two nucleosides were randomly assigned in a 1: 1 fashion to either stop taking their nucleosides or continue taking their nucleosides. All visits for the 48-week study were completed by June 2006. The baseline characteristics were similar in the two study groups (Table 1). Patients included in the trial were receiving treatment with lopinavir-ritonavir mostly as their first or second protease inhibitor and had maintained HIV virological suppression for a median time of 18 months. Prior to randomization, the most common nucleosides combinations used along with lopinavir-ritonavir were zidovudine plus lamivudine and stavudine plus lamivudine. At 48 weeks 94 patients in the monotherapy group and 88 patients in the triple therapy group were still in the study. The disposition of the two treatment groups is shown in Fig. 1. Of the 205 randomized patients, seven (three in the monotherapy and four in the triple therapy group) were not dosed due to consent withdrawal. At 48 weeks, 94/100 subjects randomized to monotherapy were still receiving lopinavir-ritonavir, 89 as monotherapy and five as triple therapy. In the triple therapy group, at 48 weeks 88/98 subjects were still receiving lopinavir-ritonavir and two nucleosides.
Response to treatment
At week 48, 94 of the 100 patients (94%) in the monotherapy group did not develop therapeutic failure compared to 88 of the 98 patients (90%) in the triple therapy group. The 95% CI for the difference between the two groups was -11.8 to 3.4% (P = 0.28), which fulfilled the pre-established criteria for non-inferiority of the monotherapy group. There were six therapeutic failures in the monotherapy group. Three were lost to follow-up. One changed randomized therapy without loss of virologic suppression. One lost virologic suppression and developed protease inhibitor resistance. One patient had loss of virologic suppression and then failed to maintain suppression after reinduction with baseline nucleosides. There were 10 therapeutic failures in the triple therapy group. Four were lost to follow up. Three had confirmed loss of virological suppression. Three discontinued randomized treatment due to adverse events. There were no significant statistical differences in the time to treatment failure between both groups by log-rank test (P = 0.29) (Fig. 2).
At week 48, six out of the 100 patients (6%) in the monotherapy group had developed confirmed loss of virological suppression compared to three out of 98 (3%) in the triple therapy group (P = 0.31). Of the six patients with loss of virological suppression in the monotherapy group, four resumed baseline nucleosides and regained virological suppression which was subsequently maintained for a median of 56 weeks (36-64). In one patient reinduction was not attempted per protocol, as genotypic testing showed lopinavir-ritonavir resistance and one patient did not maintain virological suppression after resuming baseline nucleosides.
In an intent-to-treat analysis, 89% of the patients in the monotherapy group and 90% of the patients in the triple therapy group achieved an HIV RNA level of < 500 HIV RNA copies/mL at week 48 (missing HIV RNA level values or change in randomized therapy, including successful reinduction with nucleosides in the monotherapy group, were considered to be > 500 HIV RNA copies/mL)[P = 0.85; Fisher's exact test, 95% CI (Triple-Monotherapy): -7.8 to 9.4]. Using the same analysis but with a cut-off of HIV RNA level of 50 copies/mL proportions were 85% for monotherapy and 90% for triple therapy [P = 0.31; Fisher's exact test, 95% CI (triple-monotherapy): -4.4 to 14].
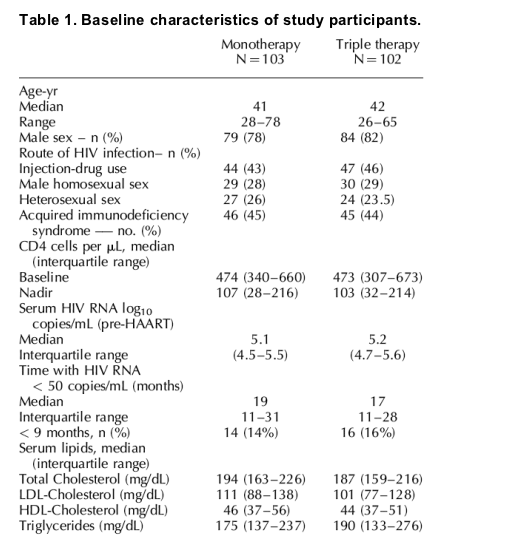
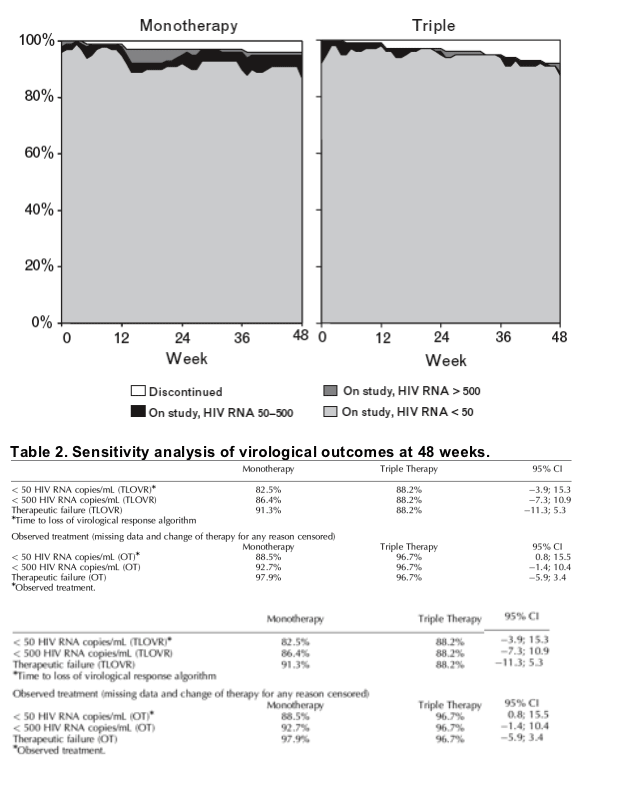
Fig. 3 shows the point prevalence of discontinuations and virologic response by as-treated analysis using both the < 50 and < 500 HIV RNA copies/mL level cut-offs. At week 48, four patients in the monotherapy group and no patient in the triple therapy group had an HIV RNA level between 50 and 500 copies/mL. Table 2 shows different sensitivity analysis for antiviral efficacy both by intention-to-treat and by an as-treated analysis. By as-treated analysis, a significantly higher proportion of patients in the triple therapy group maintained HIV RNA < 50 HIV RNA copies/mL after 48 weeks of follow-up [97% versus 89% P = 0.0496; Fisher's exact test, 95% CI (triple monotherapy), 0.8-15.5]. There were 15 patients with transitory viral loads > 50 copies/mL (blips) in the monotherapy group and 13 in the triple therapy group.
Fig. 3. Point prevalence of discontinuations and virologic response by as-treated analysis using both the less than 50 and less than 500 HIV RNA copies/mL level cutoffs. These graphs show the weekly percentage of patients in each viral load stratum. Every viral load remains in the same stratum until it is repeated or until discontinuation.
Immunologic changes
The mean increase from baseline in CD4 cell counts at week 48 was 65 cells/μL for the monotherapy group and 31cells/μL for the triple therapy group (P = 0.31; Mann-Whitney U test).
Adverse events
Study drug-related adverse events of at least moderate severity occurred in three patients in the triple therapy group (3%) and none (0%) in the monotherapy group (P = 0.08). The three adverse events in the triple therapy group were diarrhoea (two patients) and insomnia. These three adverse events resulted in treatment discontinuation.
Laboratory abnormalities
In both treatment groups there were no statistically significant changes from baseline in fasting total cholesterol, high-density lipoprotein cholesterol or triglycerides. Grade 3 or 4 hypertriglyceridaemia was seen in three patients (3%) in each group (P = 0.99). Grade 3 or 4 hypercholesterolemia was seen in 10 patients (10%) in the monotherapy group and four patients (4%) in the triple therapy group (P = 0.1). Grade 3 or 4 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevations were seen in four patients (4%) in the monotherapy group and in two patients (2%) in the triple therapy group. Of the six patients with grade 3 or 4 AST/ALT elevations, five were coinfected with hepatitis C virus. No patient discontinued the study because of elevated lipid or aminotransferase levels.
Genotypic analysis
There were 15 patients (11 in the monotherapy group, four in the triple therapy group) who qualified for genotypic testing due to a HIV RNA > 500 HIV RNA copies/mL. Protease inhibitor associated mutations were detected in three subjects, two (2%) in the monotherapy group, and one (1%), in the triple group (P = 0.56; Fisher exact test). All three subjects had exhibited more than one episode of viraemia > 500 copies/mL. Reverse transcriptase mutations were detected in two subjects, one in the monotherapy group and one in the triple therapy group.
At week 16, subject #VN04 on monotherapy showed protease mutations 10F and 46I. By phenotypic testing the fold change in 50% inhibitory concentration (IC50) to lopinavir-ritonavir was 2.6 (fold change cut-off for normal susceptible range, 1.7). The patient was reinduced with abacavir plus didanosine and reached < 50 HIV RNA copies/mL at week 24. At week 36 there was a new loss of virological suppression and genotypic testing showed protease mutations 10F, 46I and 82A. By phenotypic testing the fold change in IC50 to lopinavir-ritonavir was 2.7. The patient was switched to tenofovir DF plus stavudine plus saquinavir-ritonavir and resuppressed to < 50 HIV RNA copies/mL. This patient died shortly after resuppresion due to complications of a previously diagnosed non-Hodgkin lymphoma.
At Week 48, subject #XC02 on monotherapy showed protease mutations 54V, 77I, 82A. By phenotypic testing the fold change in IC50 to lopinavir-ritonavir was 0.7. After viral failure was switched to tenofovir DF plus emtricitabine plus saquinavir/ritonavir and remains suppressed to < 50 HIV RNA copies/mL 20 weeks after switch.
At Week 36, subject #DO34 on monotherapy had an isolated viral load > 500 copies/mL and showed reverse transcriptase mutations 41L, 74V, 210W, 215Y, without mutations in the protease gene (phenotypic testing not performed). Subject #D034 re-suppressed to <50 copies/mL while remaining on lopinavir-ritonavir monotherapy.
At Week 24, subject #DO05 receiving triple therapy showed reverse transcriptase mutations 41L, 74V, 184V, 210W, 211K, 215Y and protease mutations 54V, 63P, 71V, 82A. By phenotypic testing the fold change in IC50 to lopinavir-ritonavir was 2.8. He was lost to follow-up after viral failure.
Adherence
Using the GEEMA adherence questionnaire 44% of patients in the monotherapy group and 43% of patients in the triple therapy group were classified as adherent both to daily number and timing of doses (P = 0.88). If the item in GEEMA adherence questionnaire related to timing of doses was excluded from the analysis then 62% of patients in the monotherapy group and 55% of patients in the triple therapy group were classified as adherent to the daily number of doses (P = 0.34). The proportion of patients missing at least 1 day of medication since the prior visit were 23% in the monotherapy group and 28% in the triple therapy group (P = 0.42). The proportion of patients with at least one missed dose of medication during the week prior to study visits were 27% in the monotherapy group and 31% in the triple therapy group (P = 0.53).
Discussion
Our data indicate that for subjects who are currently suppressed while receiving lopinavir-ritonavir and two nucleosides, lopinavir-ritonavir monotherapy followed by reintroduction of nucleosides as needed is a therapeutic strategy as effective as continuing triple therapy, fulfilling the pre-established criteria for non-inferiority. The majority of patients who experienced a loss of virologic suppression while on lopinavir-ritonavir monotherapy had no evidence of resistance mutations in the protease gene and were able to resuppress and maintain suppression after resumption of baseline nucleosides. Importantly detection of resistance in the protease gene was uncommon and of little or no clinical significance. It should be noted that these results have been achieved without the need of a very intensive follow-up of the patients. In fact, during most of the trial patients visits were performed every 3 months.
Our trial shows that a substantial proportion of suppressed patients on triple therapy can maintain suppression with lopinavir-ritonavir monotherapy. This is in contrast to previous clinical trials [5-7] that attempted the use of a single antiretroviral drug for maintenance of virologic control. These findings suggest that a single antiretroviral, with a high genetic barrier to resistance, such as lopinavir-ritonavir, could be sufficient to maintain control of HIV replication and therefore challenge the notion that triple drug regimens are an absolute prerequisite for successful anti-HIV therapy.
The results of our trial also show that episodes of low level, intermittent viral replication are slightly more common in patients receiving monotherapy than in patients receiving triple therapy. Insufficient antiretroviral potency of lopinavir-ritonavir monotherapy could be an explanation of this finding. However, using an ultrasensitive (3-50 copies/mL) PCR assay, we have shown [16] that the level of persistent plasma viral load < 50 copies/mL does not increase after simplification of triple antiretroviral therapy to lopinavir-ritonavir monotherapy. Consequently, it is unlikely that insufficient antiviral potency of monotherapy could account for the observed difference in low level viraemia between monotherapy and triple therapy.
Drug adherence might be an important factor to explain the higher incidence of low-level viraemia in the monotherapy group. Adherence scores were not significantly different between groups during the trial. Less than half of the patients in both groups were classified as fully adherent by the GEEMA questionnaire. However, it should be emphasized that this questionnaire might overestimate the level of non-adherence because it gives significant weight to the question related to timing of doses. Although recent data suggest that 95% adherence level might not be absolutely predictive of virological efficacy [17] when lopinavir-ritonavir is used along with two nucleosides, our results suggest that a high level of adherence might be needed when patients receive lopinavir-ritonavir monotherapy. We hypothesize that the short-term consequences of intermittent non-adherence might have a more negative impact in patients receiving lopinavir-ritonavir monotherapy than in patients receiving triple therapy. Since lopinavir-ritonavir has a short terminal half-life [9] it is reasonable to postulate that patients missing doses of lopinavir-ritonavir monotherapy have a greater risk of virological rebound than patients receiving lopinavir-ritonavir and two nucleosides which are characterized by a long intracellular half-life. It would be interesting to evaluate if interventions that can have a benefit in adherence, such as once-daily administration of lopinavir-ritonavir or the new meltrex formulation of lopinavir-ritonavir could improve the results seen in our trial.
One limitation of our study is that there were inconsistencies in the sensitivity analyses of virological outcomes. Although the monotherapy group met the non-inferiority criteria for the primary endpoint, the analysis by observed treatment with a viraemia cut-off of 50 HIV-RNA copies/mL did not prove the non-inferiority of the monotherapy group. This result supports the notion that, in general, triple therapy has a higher probability of maintaining complete viral suppression than does monotherapy. However it should be noted that, as happened in our pilot trial, patients receiving monotherapy who had low level viraemia could be successfully reinduced by adding nucleosides again, without loss of therapeutic options. In our opinion this result supports our decision, when we designed the study, of not considering successful reinductions as failures for the primary endpoint. Nevertheless we acknowledge that longer follow-up of reinduced patients is clearly needed to completely rule out negative consequences of a period of low level viraemia in patients receiving lopinavir-ritonavir monotherapy. Finally it should be acknowledged that it is possible that patients in the triple therapy group who experienced confirmed virological rebound could have resuppressed spontaneously without needing a change in therapy.
Another limitation is that we could not show an improvement in adverse events or laboratory abnormalities in patients receiving monotherapy. However, because our study included patients who were already tolerating a stable antiretroviral regimen, the probability of showing differences in adverse events was a priori low. It is possible that longer follow-up is needed to demonstrate differences in adverse events. In addition recent data [18] have shown that monotherapy with lopinavir-ritonavir prevents development of lipoatrophy, an adverse event which was not been specifically evaluated in our study.
Lopinavir-ritonavir monotherapy has been also studied as initial treatment for antiretroviral naive patients [19] and as maintenance therapy after patients have achieved viral suppression for at least 3 months with triple therapy [20]. In each of these two studies the incidence of low level viral replication was approximately double that in our trial. In our study, all patients had maintained viral suppression for at least 6 months prior to receiving lopinavir-ritonavir monotherapy. This suggests that this period of viral suppression may be a relevant factor to predict the success of lopinavir-ritonavir monotherapy.
Being able to treat HIV infection with a single antiretroviral has important implications. Worldwide a significant barrier to treatment of HIV is the cost of antiretrovirals. By decreasing this cost, boosted protease inhibitor monotherapy has the potential to increase access to anti-HIV treatment [21]. Additionally, treating with fewer drugs means avoiding drug related toxicities and resistance to drugs not being taken. Nucleoside sparing regimens might have also an important role in settings such as the treatment of HIV-HCV coinfection in which undesirable interactions with ribavirin should be avoided. Finally although not directly applicable, our results give some support to the use of lopinavir-ritonavir monotherapy after failure of first line regimens in the underdeveloped world.
In conclusion, in patients who have achieved virological suppression for more than 6 months after receiving triple drug therapy our study supports the use of lopinavir-ritonavir monotherapy followed by reinduction with nucleosides if virological suppression is lost. Since episodes of low-level viral rebound were more frequent in the monotherapy group further studies and longer follow-up of patients treated with lopinavir-ritonavir monotherapy are needed before this strategy can be widely recommended.
|
|
| |
| |
|
|
|