| |
VAT (belly fat) is Increased in HIV+ Men, New Study Reports - Relation of Body Composition to Body Mass Index in HIV-Infected Patients With Metabolic Abnormalities
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes:Volume 47(2)1 February 2008pp 174-184
Joy, Tisha MD*; Keogh, Hester M RDSA*; Hadigan, Colleen MD*; Dolan, Sara E RN PhD(c)*; Fitch, Kathleen FNP*; Liebau, James NP*; Johnsen, Stine MD, PhD*; Lo, Janet MD*; Grinspoon, Steven K MD*
From the *Program in Nutritional Metabolism, Massachusetts General Hospital and Harvard Medical School, Boston, MA; and the National Institutes of Health, Bethesda, MD.
"....We did not follow patients longitudinally to determine changes in adipose distribution over time..... Despite these limitations, these data provide new information on the relation of body composition to BMI among HIV-infected patients....
.....VAT was significantly higher and SAT was significantly lower among HIV-infected compared with non-HIV-infected men in the normal weight and overweight categories but not in the obese category..... The difference in VAT was most significant among those in the normal weight category (104.6 ± 58.5 cm2 vs. 57.3 ± 25.5 cm2; P = 0.0004 [HIV-infected vs. controls]), whereas the difference in SAT was most significant among those in the overweight category (178.5 ± 85.6 cm2 vs. 242.2 ± 60.0 cm2; P = 0.0006 [HIV-infected vs. controls])....
.... we have demonstrated differences in central and peripheral fat depots in relation to BMI....Loss of extremity fat was the most consistent finding, but increased VAT was also observed relative to control subjects among HIV-infected men and women. The differences in VAT were most obvious among normal and overweight subjects. Gender differences in abdominal SAT accumulation were observed, with preservation of SAT among HIV-infected women relative to control subjects.....
....Our study demonstrated the significant presence of peripheral lipoatrophy among HIV-infected compared with non-HIV-infected subjects, supporting the findings of the FRAM study and others.....
.....VAT (belly fat) was increased among HIV-infected men in the normal and overweight categories compared with control subjects. Simultaneous increases in VAT and decreases in SAT may help to explain the observation that trunk fat per se was not different between HIV-infected and non-HIV-infected men.... obesity alone does not predict increased visceral adiposity among HIV-infected subjects.... Trunk/total extremity fat did not increase with BMI among HIV-infected men but did increase with BMI among non-HIV-infected men
.... Among HIV-infected men, abdominal SAT was significantly lower compared with non-HIV-infected men by an average difference of 21 cm2....
..... Among HIV-infected women, trunk fat was increased by approximately 0.67 kg compared with non-HIV-infected female control subjects...."
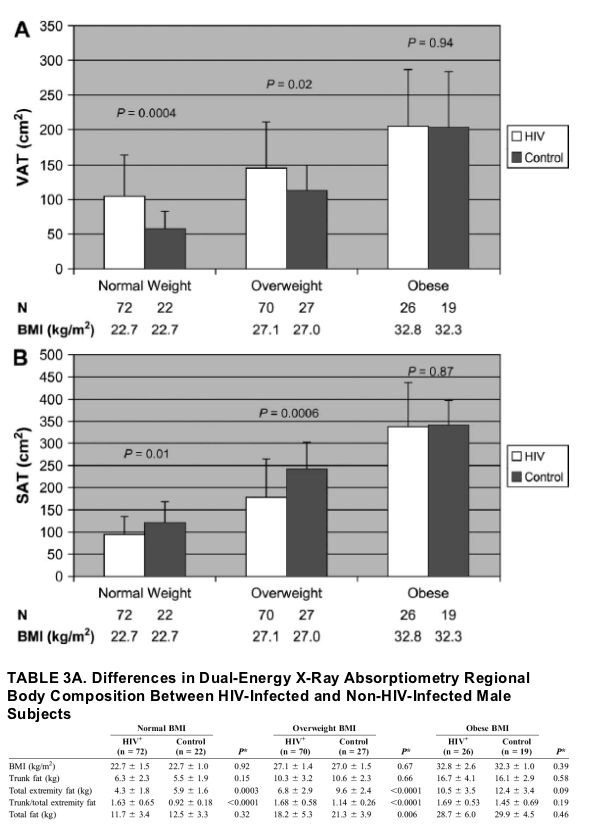
FIGURE 3. A, VAT based on BMI class among HIV-infected and control male subjects. Results are expressed as mean ± standard deviation. The white bar denotes HIV-infected subjects, whereas the black bar denotes non-HIV-infected controls. BMI categories were defined using WHO criteria17 as follows: normal weight = BMI 18.5 to 24.9, overweight = BMI 25.0 to 29.9, and obese = BMI ≥30.0. P values were derived from ANOVA testing to determine differences between HIV-infected and non-HIV-infected subjects. B, SAT based on BMI class among HIV-infected and control male subjects. Results are expressed as mean ± standard deviation. The white bar denotes HIV-infected subjects, whereas the black bar denotes non-HIV-infected controls. BMI categories were defined using WHO criteria17 as follows: normal weight = BMI 18.5 to 24.9, overweight = BMI 25.0 to 29.9, and obese = BMI ≥30.0. P values were derived from ANOVA testing to determine differences between HIV-infected and non-HIV-infected subjects.
Abstract
Objective: To determine visceral adiposity (VAT), subcutaneous adiposity (SAT), and regional body adipose differences between HIV-infected and non-HIV-infected subjects in relation to body mass index (BMI) and World Health Organization BMI categories.
Design, Setting, and Participants: Analyses were conducted of 306 HIV-infected and 107 community-derived HIV-negative subjects evaluated for metabolic studies between 1999 and 2006. Analyses were stratified by gender. Additional analyses were performed stratifying subjects by metabolic syndrome status.
Results:
HIV-infected men and women demonstrated decreased total extremity fat by 1.1 kg and 0.85 kg, respectively, relative to non-HIV-infected control subjects.
VAT was increased among HIV-infected men and women in the normal (18.5 to 24.9 kg/m2) and overweight (25.0 to 29.9 kg/m2) categories relative to control subjects but not among those in the obese category (≥30.0 kg/m2).
In contrast, abdominal SAT was reduced among HIV-infected men in the normal and overweight categories but was similar among HIV-infected women and control subjects in these categories.
Abdominal SAT was increased among HIV-infected women in the obese category relative to control subjects. Similar results were obtained limiting the analysis to HIV-infected (n = 204) and control subjects (n = 89) without the metabolic syndrome.
Conclusions: Peripheral lipoatrophy is a consistent finding among HIV-infected men and women with metabolic abnormalities. Relative increases in VAT are most pronounced among male and female HIV-infected subjects in the normal weight and overweight categories. Gender differences in abdominal SAT accumulation are observed, with preservation of SAT among HIV-infected women relative to control subjects.
The use of highly active antiretroviral therapy (HAART) has been associated with the development of metabolic complications, including dyslipidemia, insulin resistance, and altered body fat distribution.1-6 Changes in adipose distribution include peripheral lipoatrophy of face and limbs and/or central lipohypertrophy in the dorsocervical, breast, and abdominal regions.7-10 More importantly, because visceral fat accumulation has been linked to the development of cardiovascular disease and type II diabetes in the non-HIV-infected population, visceral adipose gain associated with HAART has raised concerns regarding long-term risk for cardiovascular disease in the HIV-infected population.11-13
Several studies have investigated changes in body composition that occur in HIV-infected individuals. The Fat Redistribution and Metabolic Change in HIV Infection (FRAM) study compared fat distribution between HIV-infected individuals and HIV-negative controls and revealed a greater degree of fat loss in peripheral and most central depots among HIV-infected men and women when compared with controls.14,15 Importantly, the FRAM study and several recent studies have shown that peripheral lipoatrophy is not linked to central lipohypertrophy in most HIV-infected individuals.6,14-16 Weight itself may influence the amount of adipose tissue present, however, and there may be differences in VAT (visceral adipose tissue), SAT (subcutaneous adipose tissue), and regional body adipose measurements within specific anthropometric categories. To our knowledge, differences in body composition between HIV-infected individuals and HIV-negative controls stratified by standard anthropometric cutoff points for body mass index (BMI) have not been studied. We therefore sought to examine the relations between VAT, SAT, or regional body adipose measurements and BMI among HIV-infected and non-HIV-infected subjects and to determine how these relations differed between these groups of subjects within the World Health Organization (WHO) BMI categories of normal weight, overweight, and obese.17
DISCUSSION
Although BMI has been used as a simple anthropometric predictor of type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease among non-HIV-infected individuals,34-37 few studies have evaluated body composition between HIV-infected and non-HIV-infected control subjects in relation to BMI. We therefore examined the relation between body composition and BMI for HIV-infected compared with non-HIV-infected subjects in linear regression modeling and used the WHO BMI criteria to determine fat distribution differences between HIV-infected and non-HIV-infected subjects within these anthropometric subgroups.17
Our study demonstrated the significant presence of peripheral lipoatrophy among HIV-infected compared with non-HIV-infected subjects, supporting the findings of the FRAM study and others.14-16,38 For any given BMI, HIV-infected male subjects demonstrated 1.1 kg less total extremity fat and HIV-infected female subjects demonstrated 0.85 kg less total extremity fat compared with their respective non-HIV-infected controls. To our knowledge, this is the first study to quantify the degree of fat loss in relation to BMI between HIV-infected and non-HIV-infected subjects stratified by gender. We have also shown that in addition to the presence of peripheral lipoatrophy among HIV-infected individuals, significant alterations in VAT, SAT, and truncal fat occur in the context of BMI within gender categories.
Among HIV-infected men, abdominal SAT was significantly lower compared with non-HIV-infected men by an average difference of 21 cm2, and the largest differences were seen among normal and overweight HIV-infected men compared with control subjects. In contrast, VAT was increased among HIV-infected men in the normal and overweight categories compared with control subjects. Simultaneous increases in VAT and decreases in SAT may help to explain the observation that trunk fat per se was not different between HIV-infected and non-HIV-infected men.
Among HIV-infected women, trunk fat was increased by approximately 0.67 kg compared with non-HIV-infected female control subjects. Similar to the observation in men, VAT was increased most among HIV-infected women in the normal and overweight categories relative to controls. In contrast to the findings among men, SAT was not different between normal weight and overweight subjects (HIV-infected subject vs. control); thus, the increased trunk fat in these categories was primarily attributable to increased VAT, whereas in the obese category, the increased trunk fat was attributable to increased SAT. Women have more total body fat than men and tend to preserve gluteal and femoral fat stores,39 which may help to explain observed differences between HIV-infected men and women in this and other studies.14,15
HIV-infected and control subjects (male and female) also demonstrated increasing VAT and SAT deposition with increasing BMI, supporting the results of other studies of non-HIV-infected individuals.40 Thus, although absolutes levels of VAT increase with increasing BMI, relative differences compared with control subjects are greatest for normal and overweight HIV-infected patients (male and female). These findings suggest that (1) obesity alone does not predict increased visceral adiposity among HIV-infected subjects relative to non-HIV-infected subjects and (2) HIV-infected subjects at relatively lower BMIs may have increased risk of metabolic complications, given the degree of visceral adiposity observed.
In the FRAM study,14,15 patients were categorized based on the presence of lipoatrophy. Among men, a trend toward more VAT and significantly more trunk fat was demonstrated in HIV-infected patients without lipoatrophy compared with those with lipoatrophy. In contrast, VAT was lower compared with non-HIV-infected controls in those with lipoatrophy. Among women, more VAT and trunk fat were seen among HIV-infected subjects without lipoatrophy compared with HIV-infected patients with lipoatrophy and with non-HIV-infected controls. Subjects were not compared in relation to BMI or within BMI categories in the FRAM study; rather, adipose tissue volume for each subject was divided by height squared and then multiplied by 1.752 to correspond to a typical height. In contrast, HIV-infected patients and controls were compared in relation to BMI and within BMI categories in our study, suggesting relatively more VAT deposition among HIV-infected patients compared with controls at lower BMIs, particularly in the normal and overweight BMI categories, for both genders. Thus, our data extend those of the FRAM study, demonstrating relative differences in fat accumulation and fat loss by BMI category between genders.
This study has a number of limitations. We assessed body composition among HIV-infected individuals with a high proportion of metabolic abnormalities. Our results therefore cannot be generalized to all HIV-infected individuals or to HIV-infected individuals with wasting. Similar results were obtained when body composition parameters were compared between HIV-infected and non-HIV-infected patients using an identical criterion of absence of NCEP/ATP III-defined metabolic syndrome, however. These data suggest that the changes in body composition among the HIV-infected patients relative to controls in this study were not significantly biased by selection of patients with a high proportion of metabolic abnormalities. We did not follow patients longitudinally to determine changes in adipose distribution over time. Finally, we were unable to analyze the respective contributions from deep and superficial subcutaneous compartments, which may help to define the specific adipose changes that are occurring among HIV-infected individuals. Despite these limitations, these data provide new information on the relation of body composition to BMI among HIV-infected patients.
In conclusion, we have demonstrated differences in central and peripheral fat depots in relation to BMI and by WHO BMI category in the comparisons of male and female HIV-infected versus control subjects. Loss of extremity fat was the most consistent finding, but increased VAT was also observed relative to control subjects among HIV-infected men and women. The differences in VAT were most obvious among normal and overweight subjects. Gender differences in abdominal SAT accumulation were observed, with preservation of SAT among HIV-infected women relative to control subjects.
METHODS
Patients and Controls
Data on body composition parameters were prospectively collected from 1999 to 2006 in 306 HIV-infected subjects participating in metabolic studies at the Massachusetts General Hospital (MGH)18-27 and 107 HIV-negative subjects simultaneously recruited from the community as controls for the HIV studies20-22,26,27 and as participants for metabolic studies in non-HIV-infected individuals.28,29 HIV-infected subjects in some18-23,25,27 but not all studies were recruited based on the presence of lipodystrophy. Among the HIV-infected patients, 70% were characterized as having lipodystrophy based on a previously used definition.19 Among studies in which HIV-infected and non-HIV-infected subjects were simultaneously recruited, subjects of similar weight were recruited. Dietary data on a subset were recently published.30 HIV-infected subjects with known wasting or evaluated for studies of AIDS wasting were not included in the analysis. HIV-infected subjects aged 18 to 60 years were recruited from newspaper advertisements, the community, and referral-based practices. For subjects receiving antiretroviral (ARV) therapy, a stable regimen for a minimum of 6 weeks before evaluation was required. Subjects in both groups were excluded if they had a history of diabetes mellitus; were receiving concurrent therapy with insulin, antidiabetic agents, glucocorticoids, growth hormone or growth hormone-releasing analogues, supraphysiologic testosterone replacement, or anabolic steroids; were current substance abusers; had a major opportunistic infection within the 6 weeks before the study; or were pregnant or breast-feeding within the past year. The HIV-negative controls were recruited through hospital and local advertisements. Other criteria, including age, medication use, and reproductive status, were similar between the HIV-infected and non-HIV-infected groups. For the HIV-infected and control groups, baseline data were obtained before any intervention. If subjects participated in more than 1 study, only data from the initial study were used. Collection of all data was approved by the Institutional Review Board at the MGH and at the Massachusetts Institute of Technology (MIT), and all participants provided informed consent.
Protocol
All subjects were studied after an overnight fast of 12 hours. Each individual had a complete medical history (including documentation of current ARV use) and a physical examination, which included measurement of height by stadiometer and weight by digital scale.
Subjects underwent total-body dual-energy x-ray absorptiometry (Hologic QDR-4500A; Hologic, Waltham, MA) to determine regional fat and lean mass.31 Cross-sectional abdominal computed tomography (CT) scans were performed, as described by Borkan et al,32 to assess distribution of SAT and VAT, respectively. A lateral scout image was obtained to identify the level of the L4 pedicle, which served as the landmark for the 1-cm single-slice image. Subjects also received a standard 75-g oral glucose tolerance test (OGTT), with glucose and insulin determinations at 30, 60, 90, and 120 minutes after the OGTT. CD4 cell count and HIV viral load as well as concentrations of glucose, insulin, cholesterol, high-density lipoprotein (HDL), and triglycerides were determined by methods described elsewhere.19
Statistical Analysis
We performed an analysis to determine the impact of BMI as a continuous variable on various body composition parameters (VAT, SAT, VAT/SAT, trunk fat, total extremity fat, and trunk/total extremity fat) in HIV-infected and non-HIV-infected subjects, stratified by gender, using linear fit modeling. Slopes were tested between the HIV-infected and non-HIV-infected groups by analysis of covariance (ANCOVA), testing for an interaction between the groups. If the slopes were parallel (ie, not statically significantly different), ANCOVA was subsequently performed to determine the difference in Y-intercepts. In a subanalysis, HIV-infected patients and controls were characterized based on National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP III) criteria for the metabolic syndrome.33 We used ANCOVA analysis in these subgroups without metabolic syndrome to determine the relation of BMI to body composition parameters among subjects meeting an identical criterion for analysis.
Subjects were also stratified by gender into 3 categories based on WHO BMI class (normal weight = BMI of 18.5 to 24.9 kg/m2, overweight = BMI of 25.0 to 29.9 kg/m2, and obese = BMI ≥30.0 kg/m2)17 to determine differences in body composition between HIV-infected and non-HIV-infected subjects within each category. Data are expressed as mean ± standard deviation except where indicated. P values were derived from the ANOVA test to determine differences between HIV-infected and control subjects, and a P value <0.05 was considered significant. All statistical analyses were performed using SAS JMP software, version 5.0.1 (SAS Institute, Cary, NC).
RESULTS
Demographics
Three hundred six HIV-infected subjects (168 male and 138 female) and 107 non-HIV-infected controls (68 male and 39 female) were evaluated. There were no statistically significant differences in age, race, or BMI between the HIV-infected and control subjects stratified by gender. HIV-infected male subjects demonstrated higher total cholesterol and triglyceride levels, lower HDL levels, higher fasting insulin and insulin areas under the curve (AUCs), and higher fasting glucose and glucose AUCs compared with male controls. HIV-infected female subjects also demonstrated lower HDL, higher fasting insulin, and higher insulin AUC levels compared with female controls (Table 1). Demographics were also similar in the subanalysis limited to HIV-infected and control subjects without the metabolic syndrome (Table 2).
Men
Significant associations between BMI and SAT, VAT, trunk fat, and total extremity fat were observed for HIV-infected and non-HIV-infected men (Fig. 1). Regression lines were parallel, and intercepts differed significantly in the comparison of the relation of SAT to BMI between the HIV-infected and non-HIV-infected groups. SAT was decreased by approximately 21 cm2 for a given BMI among the male HIV-infected subjects compared with non-HIV-infected subjects. In contrast, the slopes of the regression lines relating VAT or VAT/SAT to BMI differed significantly between the HIV-infected and non-HIV-infected groups.
Trunk fat was not increased for a given BMI in male HIV-infected versus non-HIV-infected subjects, but total extremity fat was lower by 1.1 kg across the range of BMI for male HIV-infected compared with non-HIV-infected subjects. Trunk/total extremity fat was increased for male HIV-infected versus non-HIV-infected subjects, but differences between HIV-infected and non-HIV-infected men decreased with increasing BMI. Trunk/total extremity fat did not increase with BMI among HIV-infected men but did increase with BMI among non-HIV-infected men (see Fig. 1). The difference in VAT was most significant among those in the normal weight category (104.6 ± 58.5 cm2 vs. 57.3 ± 25.5 cm2; P = 0.0004 [HIV-infected vs. controls]), whereas the difference in SAT was most significant among those in the overweight category (178.5 ± 85.6 cm2 vs. 242.2 ± 60.0 cm2; P = 0.0006 [HIV-infected vs. controls]) (Fig. 3).
Analyses of HIV-infected patients and controls without metabolic syndrome as defined by NCEP/ATP III guidelines revealed similar results to the primary findings among all the subjects (Fig. 2).
Seventy-two (43%) male HIV-infected subjects and 22 (32%) male control subjects were of normal weight, 70 (42%) male HIV-infected subjects and 27 (40%) male control subjects were overweight, and 26 (15%) male HIV-infected subjects and 19 (28%) male control subjects were obese using WHO criteria. VAT was significantly higher and SAT was significantly lower among HIV-infected compared with non-HIV-infected men in the normal weight and overweight categories but not in the obese category. The difference in VAT was most significant among those in the normal weight category (104.6 ± 58.5 cm2 vs. 57.3 ± 25.5 cm2; P = 0.0004 [HIV-infected vs. controls]), whereas the difference in SAT was most significant among those in the overweight category (178.5 ± 85.6 cm2 vs. 242.2 ± 60.0 cm2; P = 0.0006 [HIV-infected vs. controls]) (Fig. 3).
Consistent with linear regression modeling, trunk fat was not increased among HIV-infected versus non-HIV-infected men for any BMI category, whereas total extremity fat was decreased in the normal and overweight categories and tended to be decreased in the obese category. Trunk/total extremity fat was therefore significantly increased in the normal and overweight categories, driven primarily by lower total extremity fat values (Table 3A).
NRTI and NNRTI use did not differ among HIV-infected men within the 3 BMI categories. The percentage of HIV-infected men receiving PIs was lower in the obese category compared with the other 2 BMI categories (48.0% PI use in obese vs. 74.2% in normal and 78.8% in overweight; P = 0.01). VAT (151.4 ± 70.4 cm2 vs. 136.2 ± 72.0 cm2; P = 0.23 [no PI vs. PI use]) and SAT (188.4 ± 119.0 cm2 vs. 162.1 ± 108.6 cm2; P = 0.19 [no PI vs. PI use]) did not differ by PI use, nor did PI use influence VAT or SAT in regression modeling accounting for BMI category (P = 0.74 for VAT and P = 0.52 for SAT).
Women
Significant associations were observed between BMI and SAT, VAT, trunk fat, and total extremity fat for HIV-infected and non-HIV-infected women (see Fig. 3). The relation of SAT to BMI was not significantly different between the HIV-infected and non-HIV-infected women. In contrast, the relations of VAT and VAT/SAT to BMI differed significantly between HIV-infected and non-HIV-infected women.
Regression lines were parallel, and intercepts differed significantly in the comparisons of trunk fat and total extremity fat to BMI between HIV-infected and non-HIV-infected women. For a given BMI, female HIV-infected subjects demonstrated increased trunk fat and decreased total extremity fat by differences of approximately 0.67 kg and 0.85 kg, respectively, compared with non-HIV-infected subjects. Trunk/total extremity fat did not increase with BMI among HIV-infected women in contrast to non-HIV-infected women (Fig. 4).
Analyses of HIV-infected patients and controls without metabolic syndrome as defined by NCEP/ATP III guidelines revealed similar results to the primary findings among all the subjects (Fig. 5).
Forty-six (33%) female HIV-infected subjects and 14 (36%) female control subjects were of normal weight, 53 (39%) female HIV-infected subjects and 13 (33%) female control subjects were overweight, and 39 (28%) female HIV-infected subjects and 12 (31%) female control subjects were obese using WHO criteria. VAT was significantly higher among HIV-infected compared with non-HIV-infected women in the normal weight and overweight categories but not in the obese category. The difference in VAT was most significant among those in the normal weight category (76.6 ± 48.9 cm2 vs. 36.7 ± 13.9 cm2; P = 0.004 [HIV-infected vs. controls]). In contrast, SAT was significantly higher among HIV-infected compared with non-HIV-infected women in the obese category (472.4 cm2 ± 113.9 vs. 400.1 ± 83.7 cm2; P = 0.048) (Fig. 6).
Trunk fat values were significantly higher in the HIV-infected women in the normal and overweight categories and tended to be higher in the obese category (see Table 3B). HIV-infected women demonstrated higher trunk fat/total extremity fat across all BMI categories.
Protease inhibitor (PI), nucleoside reverse transcriptase inhibitor (NRTI), and nonnucleoside reverse transcriptase inhibitor (NNRTI) use was similar among the HIV-infected women across the 3 BMI categories. VAT (118.1 ± 64.0 cm2 vs. 120.4 ± 66.9 cm2; P = 0.85 [no PI vs. PI use]) and SAT (300.4 ± 181.0 cm2 vs. 278.2 ± 139.1 cm2; P = 0.46 [no PI vs. PI use]) did not differ by PI use, nor did PI use influence VAT or SAT in regression modeling accounting for BMI category (P = 0.76 for VAT and P = 0.53 for SAT).
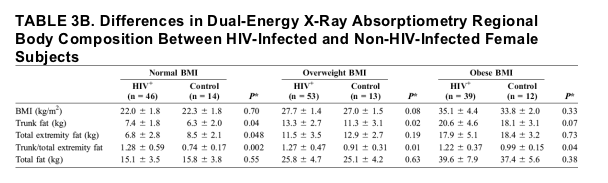
FIGURE 6. A, VAT based on BMI class among HIV-infected and control female subjects. Results are expressed as mean ± standard deviation. The white bar denotes HIV-infected subjects, whereas the black bar denotes non-HIV-infected controls. BMI categories were defined using WHO criteria17 as follows: normal weight = BMI 18.5 to 24.9, overweight = BMI 25.0 to 29.9, and obese = BMI ≥30.0. P values were derived from ANOVA testing to determine differences between HIV-infected and non-HIV-infected subjects. B, SAT based on BMI class among HIV-infected and control female subjects. Results are expressed as mean ± standard deviation. The white bar denotes HIV-infected subjects, whereas the black bar denotes non-HIV-infected controls. BMI categories were defined using WHO criteria17 as follows: normal weight = BMI 18.5 to 24.9, overweight = BMI 25.0 to 29.9, and obese = BMI ≥30.0. P values were derived from ANOVA testing to determine differences between HIV-infected and non-HIV-infected subjects.
DISCUSSION
Although BMI has been used as a simple anthropometric predictor of type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease among non-HIV-infected individuals,34-37 few studies have evaluated body composition between HIV-infected and non-HIV-infected control subjects in relation to BMI. We therefore examined the relation between body composition and BMI for HIV-infected compared with non-HIV-infected subjects in linear regression modeling and used the WHO BMI criteria to determine fat distribution differences between HIV-infected and non-HIV-infected subjects within these anthropometric subgroups.17
Our study demonstrated the significant presence of peripheral lipoatrophy among HIV-infected compared with non-HIV-infected subjects, supporting the findings of the FRAM study and others.14-16,38 For any given BMI, HIV-infected male subjects demonstrated 1.1 kg less total extremity fat and HIV-infected female subjects demonstrated 0.85 kg less total extremity fat compared with their respective non-HIV-infected controls. To our knowledge, this is the first study to quantify the degree of fat loss in relation to BMI between HIV-infected and non-HIV-infected subjects stratified by gender. We have also shown that in addition to the presence of peripheral lipoatrophy among HIV-infected individuals, significant alterations in VAT, SAT, and truncal fat occur in the context of BMI within gender categories.
Among HIV-infected men, abdominal SAT was significantly lower compared with non-HIV-infected men by an average difference of 21 cm2, and the largest differences were seen among normal and overweight HIV-infected men compared with control subjects. In contrast, VAT was increased among HIV-infected men in the normal and overweight categories compared with control subjects. Simultaneous increases in VAT and decreases in SAT may help to explain the observation that trunk fat per se was not different between HIV-infected and non-HIV-infected men.
Among HIV-infected women, trunk fat was increased by approximately 0.67 kg compared with non-HIV-infected female control subjects. Similar to the observation in men, VAT was increased most among HIV-infected women in the normal and overweight categories relative to controls. In contrast to the findings among men, SAT was not different between normal weight and overweight subjects (HIV-infected subject vs. control); thus, the increased trunk fat in these categories was primarily attributable to increased VAT, whereas in the obese category, the increased trunk fat was attributable to increased SAT. Women have more total body fat than men and tend to preserve gluteal and femoral fat stores,39 which may help to explain observed differences between HIV-infected men and women in this and other studies.14,15
HIV-infected and control subjects (male and female) also demonstrated increasing VAT and SAT deposition with increasing BMI, supporting the results of other studies of non-HIV-infected individuals.40 Thus, although absolutes levels of VAT increase with increasing BMI, relative differences compared with control subjects are greatest for normal and overweight HIV-infected patients (male and female). These findings suggest that (1) obesity alone does not predict increased visceral adiposity among HIV-infected subjects relative to non-HIV-infected subjects and (2) HIV-infected subjects at relatively lower BMIs may have increased risk of metabolic complications, given the degree of visceral adiposity observed.
In the FRAM study,14,15 patients were categorized based on the presence of lipoatrophy. Among men, a trend toward more VAT and significantly more trunk fat was demonstrated in HIV-infected patients without lipoatrophy compared with those with lipoatrophy. In contrast, VAT was lower compared with non-HIV-infected controls in those with lipoatrophy. Among women, more VAT and trunk fat were seen among HIV-infected subjects without lipoatrophy compared with HIV-infected patients with lipoatrophy and with non-HIV-infected controls. Subjects were not compared in relation to BMI or within BMI categories in the FRAM study; rather, adipose tissue volume for each subject was divided by height squared and then multiplied by 1.752 to correspond to a typical height. In contrast, HIV-infected patients and controls were compared in relation to BMI and within BMI categories in our study, suggesting relatively more VAT deposition among HIV-infected patients compared with controls at lower BMIs, particularly in the normal and overweight BMI categories, for both genders. Thus, our data extend those of the FRAM study, demonstrating relative differences in fat accumulation and fat loss by BMI category between genders.
This study has a number of limitations. We assessed body composition among HIV-infected individuals with a high proportion of metabolic abnormalities. Our results therefore cannot be generalized to all HIV-infected individuals or to HIV-infected individuals with wasting. Similar results were obtained when body composition parameters were compared between HIV-infected and non-HIV-infected patients using an identical criterion of absence of NCEP/ATP III-defined metabolic syndrome, however. These data suggest that the changes in body composition among the HIV-infected patients relative to controls in this study were not significantly biased by selection of patients with a high proportion of metabolic abnormalities. We did not follow patients longitudinally to determine changes in adipose distribution over time. Finally, we were unable to analyze the respective contributions from deep and superficial subcutaneous compartments, which may help to define the specific adipose changes that are occurring among HIV-infected individuals. Despite these limitations, these data provide new information on the relation of body composition to BMI among HIV-infected patients.
In conclusion, we have demonstrated differences in central and peripheral fat depots in relation to BMI and by WHO BMI category in the comparisons of male and female HIV-infected versus control subjects. Loss of extremity fat was the most consistent finding, but increased VAT was also observed relative to control subjects among HIV-infected men and women. The differences in VAT were most obvious among normal and overweight subjects. Gender differences in abdominal SAT accumulation were observed, with preservation of SAT among HIV-infected women relative to control subjects.
|
|
| |
| |
|
|
|