| |
Lipodystrophy and weight changes: data from the Swiss HIV Cohort Study, 2000-2006
|
| |
| |
HIV Medicine (OnlineEarly Articles). Jan 2008
A Nguyen1, A Calmy11Infectious Disease/HIV Unit, University Hospital Geneva, Geneva, Switzerland, V Schiffer1, E Bernasconi2, M Battegay3, M Opravil44Division of Infectious Diseases, University Hospital Zurich, Zurich, Switzerland, J-M Evison5, P Schmid77Infectious Disease Unit, Hospital St-Gall, St-Gall, Switzerland and, T Perneger8, B Hirschel1
1Infectious Disease/HIV Unit, University Hospital Geneva, Geneva, Switzerland,
2Infectious Disease Service, Hospital Lugano, Lugano,
Switzerland, 3Division of Infectious Diseases, University Hospital
Basel, Basel, Switzerland, 4Division of Infectious Diseases, University Hospital Zurich, Zurich, Switzerland, 5Infectious Disease Service,
University Hospital Bern, Bern, Switzerland, 6Infectious
Disease Service, University Hospital Lausanne, Lausanne, Switzerland, 7Infectious Disease Unit, Hospital St-Gall, St-Gall, Switzerland
and 8Quality of Care Service, University Hospital Geneva, Geneva, Switzerland
Abstract
Background and Objectives
Combination antiretroviral therapy (cART) is changing, and this may affect the type and occurrence of side effects. We examined the frequency of lipodystrophy (LD) and weight changes in relation to the use of specific drugs in the Swiss HIV Cohort Study (SHCS).
Methods
In the SHCS, patients are followed twice a year and scored by the treating physician as having 'fat accumulation', 'fat loss', or neither. Treatments, and reasons for change thereof, are recorded. Our study sample included all patients treated with cART between 2003 and 2006 and, in addition, all patients who started cART between 2000 and 2003.
Results
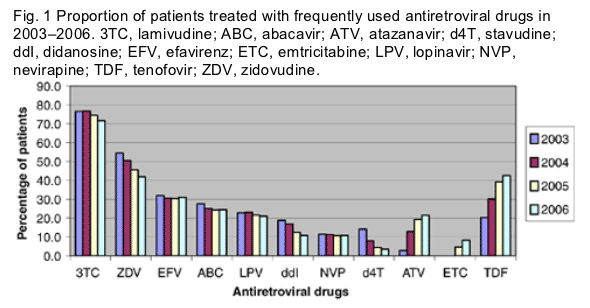
From 2003 to 2006, the percentage of patients taking stavudine, didanosine and nelfinavir decreased, the percentage taking lopinavir, nevirapine and efavirenz remained stable, and the percentage taking atazanavir and tenofovir increased by 18.7 and 22.2%, respectively.
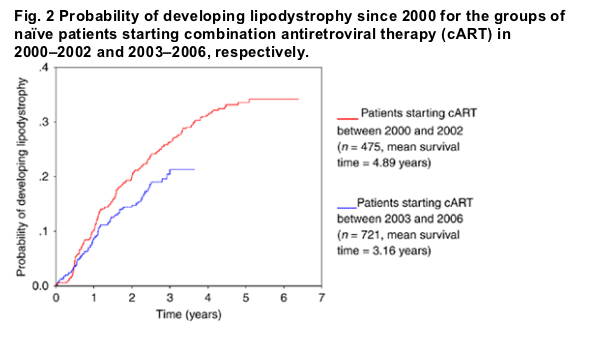
In life-table Kaplan-Meier analysis, patients starting cART in 2003-2006 were less likely to develop LD than those starting cART from 2000 to 2002 (P<0.02).
LD was quoted as the reason for treatment change or discontinuation for 4% of patients on cART in 2003, and for 1% of patients treated in 2006 (P for trend <0.001).
In univariate and multivariate regression analysis, patients with a weight gain of ≥5 kg were more likely to take lopinavir or atazanavir than patients without such a weight gain [odds ratio (OR) 2, 95% confidence interval (CI) 1.3-2.9, and OR 1.7, 95% CI 1.3-2.1, respectively].
Conclusions
LD has become less frequent in the SHCS from 2000 to 2006. A weight gain of more than 5 kg was associated with the use of atazanavir and lopinavir.
Introduction
Combination antiretroviral therapy (cART) against HIV infection has diminished AIDS-related morbidity and mortality [1]. Suppression of viral replication to below the detection level has become a treatment goal that can also be reached for patients with triple class treatment failure.
However, patients on cART often develop long-term side effects, particularly lipodystrophy (LD) syndrome, a combination of fat atrophy (in the limbs, buttocks and face) and visceral fat accumulation, with or without lipid and glucose metabolism disturbances [2-4]. The definition of 'LD' remains a challenge [39]. Proposals for a case definition have been put forward, but necessitate dual energy x-ray absorptiometry (DEXA) and computed tomography (CT) scans [3-7]; while invaluable for small-scale studies, they are not practical for routine use in patients. Instead, larger cohorts and epidemiological studies most often rely only on the subjective impressions of patients and/or physicians [8].
LD may decrease compliance with therapy for aesthetic reasons [9], but also may be associated with an increased risk of cardiovascular diseases [5,9], insulin resistance [10,11], and diabetes [6,12,13].
The aetiology of LD in HIV-infected patients is still unclear [3-7]. LD was initially attributed to prolonged therapy with protease inhibitors (PIs) [14-16], but all current drug classes have been involved in LD [17-19].
Lipoatrophy is related to the mitochondrial toxicity of nucleoside reverse transcriptase inhibitors (NRTIs), whose action is not limited to inhibition of reverse transcription, but extends to mitochondrial DNA polymerase ƒÁ, the main enzyme involved in replication of mitochondrial DNA. Inhibition of this enzyme is associated with abnormal mitochondrial morphology and may induce apoptosis of adipocytes and lipoatrophy [20,21]. In contrast, the pathogenesis of fat accumulation is not clear. Proposed mechanisms abound: highly active antiretroviral therapy (HAART) may have an impact on cytokines and cytokine receptors [22] and appetite regulators [23], as well as on the differentiation and functions of the adipocytes [20,21,24] and growth hormone metabolism [25,26]. Another theory holds that abdominal and visceral fat accumulation is a consequence of lipoatrophy, because the atrophic fatty tissues are unable to take up fat from the plasma [4]. Of note, a pure phenotype of fat atrophy or fat accumulation is only seen in 10% of patients. The paradox that central fat increases while subcutaneous fat decreases can be explained by the difference between visceral fat (mainly comprising brown adipocytes) and subcutaneous fat (comprising mainly white adipocytes): thus exposure to antiretrovirals may affect subcutaneous adipose tissue differently from visceral adipose tissue [27]. Because HAART entails the combined use of several drugs, it has been difficult to link the various manifestations of LD to a particular molecule. Cross-sectional and prospective studies demonstrated that stavudine (d4T) was the drug most frequently associated with fat atrophy [28,29]. Other risk factors include race, age and HIV disease severity for fat atrophy, and race, sex and sedentary lifestyle for fat accumulation [4].
The present paper attempts to analyse these data in relation to the type of cART administered. It focuses on the period from 2003 to 2006, coincident with the introduction of atazanavir (ATV) and the introduction of new NRTIs.
Patients and methods
The Swiss HIV Cohort Study (SHCS) is a nationwide prospective study based on voluntary participation of persons infected with HIV-1. The rationale, organization and baseline characteristics of the study have been described elsewhere in detail [30], and a continuously updated description can be found at the Swiss HIV Cohort website [31].
LD is coded based on the subjective impression of the treating physician as 'fat accumulation' or 'fat loss', and is routinely recorded 6-monthly in the SHCS database. More objective measurements, such as DEXA scans or computed tomography (CT) scans, were not available for this study.
Incidence of LD in patients starting cART
All study participants who had previously been antiretroviral-naive, but who started cART between 1 January 2000 and 31 December 2006, were eligible for inclusion. The time to the occurrence of 'fat loss' or 'fat accumulation', or a treatment change because of LD noted at one of the 6-monthly cohort visits, was measured according to Kaplan-Meier. Two groups were compared with the log-rank test: patients starting cART between 2000 and 2002 and patients starting cART between 2003 and 2006.
Prevalence of weight changes, fat loss and fat accumulation in patients on cART
To establish how frequently antiretroviral treatment was changed because of 'fat loss' or 'fat accumulation', we included all patients on cART with at least one follow-up visit between 2003 and 2006. The number of patients on cART with a discontinuation or change of treatment because of 'fat loss' or 'fat accumulation' was determined and related to the total number of patients on cART during the same year, considering only the first change or discontinuation in that year. A P-trend analysis for the prevalence of discontinuation or change in general, and the prevalence of discontinuation or change in particular because of 'fat loss or fat accumulation', was performed.
Analysis of factors associated with weight gain, weight loss, fat loss and fat accumulation
In order to analyse factors associated with gain of weight, patients who gained ≥5 kg between two consecutive visits were compared with patients who did not have such gain of weight. In order to exclude from consideration the weight gain corresponding to correction of AIDS-related cachexia, we included in this analysis only patients with a baseline body mass index (BMI) above 20 for men and above 18 for women.
Factors associated with a weight gain ≥5 kg between the first two follow-up cohort visits between 2003 and 2006 were examined using univariate analysis followed by multivariate logistic regression. Gender, ethnic origin, diabetes status based on the guidelines of the American Diabetes Association (ADA) [32], smoking status and cART (current exposure, i.e. cART received during the period when weight changed or fat loss/accumulation occurred) were entered as categorical variables; for age, BMI, CD4 cell count, RNA viral load, plasma cholesterol (mmol/L), and triglyceride (mmol/L), quartiles were compared (see Table 1). The baseline was defined as the first visit of the first two follow-up cohort visits between 2003 and 2006.
The multivariate model was constructed using the forward stepwise approach, including covariates that remained significant at the 0.1 level in univariate analysis. We repeated the analysis for factors associated with weight loss, fat accumulation and fat loss.
Analyses were performed using the spss version 11.0 for Windows statistical software package (SPSS Inc., Chicago, IL, USA).
Results
Use of antiretroviral drugs in SHCS, 2003-2006
In each year from 2000 to 2006, between 5203 and 6016 patients had a follow-up visit in the SHCS. Figure 1 shows the proportion of patients treated with the 10 most frequently used drugs, plus d4T. Between 2003 and 2006, use of zidovudine (ZDV), lamivudine (3TC), didanosine (ddI) and d4T declined, use of lopinavir (LPV), abacavir (ABC), efavirenz (EFV) and nevirapine (NVP) remained stable, and use of ATV, emtricitabine (ETC) and tenofovir (TDF) increased by 18.7, 8.2 and 22.2%, respectively.
Incidence of LD in treatment-naive patients
Figure 2 shows the likelihood of occurrence of LD in patients who were cART-naive, and started treatment between 2000 and 2006. Patients starting treatment in 2003-2006 were significantly less likely to experience LD than those starting between 2000 and 2002 (log rank=0.02). From 2000 to 2002, most patients were started on 3TC (96.9%), ZDV (88.5%), d4T (4.2%), TDF (0.8%), EFV (38.1%), nelfinavir (NFV) (28.8%) and LPV (13.8). From 2003 to 2006, the corresponding percentages were 83.7% for 3TC, 10.8% for ETC, 64.2% for ZDV, 0.7% for d4T, 30.5% for TDF, 41.8% for EFV, 3.8% for NFV, 38.5% for LPV and 7.5% for ATV. Overall, the proportion of patients initiating cART with ATV, ritonavir-boosted LPV (LPV/r) or TDF was higher in the 2003-2006 period than in the 2000-2002 period.
Treatment changes because of LD
A total of 5777 patients were followed in the SHCS in 2003, and 6016 during 2006. Baseline characteristics are summarized in Table 1: 32% of the patients were female, and they had a median age of 42.5 years [interquartile range (IQR) 37.5-48.5 years]. The number of patients treated with cARTs was 5269 (91.2%) in 2003 and 5530 (91.9%) in 2006. In 2003, 44% underwent a change or discontinuation of treatment, while in 2006 the proportion decreased to 17% (P<0.001). LD was quoted as the reason for treatment change or discontinuation for 4% of patients on cARTs in 2003, but for only 1% of patients treated in 2006 (P for trend <0.001).
10.4% of patients were diagnosed with only fat loss and 12.3% with only fat accumulation. Overall, 33.9% had a diagnosis of either fat loss and fat accumulation or fat loss or fat accumulation.
We determined the proportion of patients who had a 'fat loss' or a 'fat accumulation' diagnosis before our baseline. 90.4% of ABC-treated patients diagnosed with fat accumulation and 93.4% of those diagnosed with fat loss had had this diagnosis for more than 2 years. For ZDV-treated patients, we observed similar results (83.1% for fat accumulation and 86.4% for fat loss).
Table 1 Baseline characteristics of patients
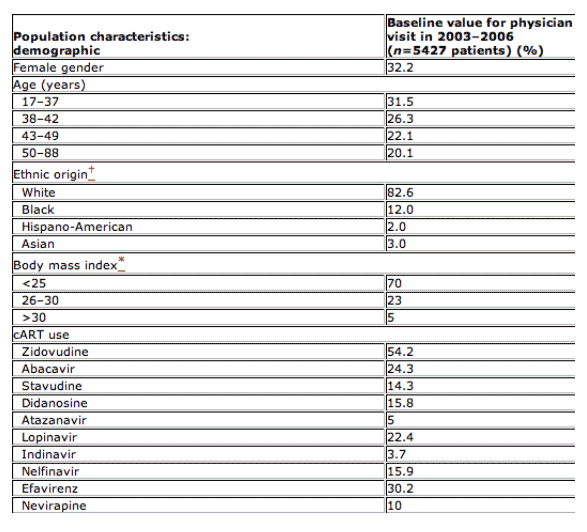
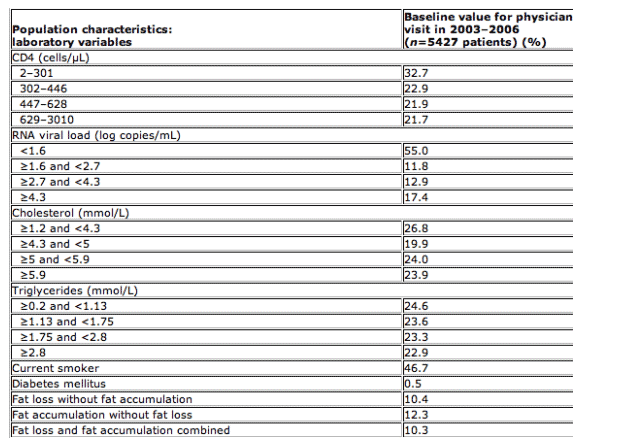
cART, combination antiretroviral therapy; *2% of population had unknown heights, thus BMI was not available; 0.4% of population had unknown ethnic origins.
Factors involved in weight changes and fat redistribution (see Tables 2 and 3) Weight changes
Weight gain of ≥5 kg
The mean BMI of our population was 23.7 kg/m2. In 2003, of the patients with eligible BMIs (>18 kg/m2), 494 (7.03%) experienced a rapid gain of weight of ≥5 kg within a 6-month period, as compared with 491 (6.49%) in 2004, 517 (6.57%) in 2005, and 222 (5.51%) in 2006 (difference not significant).
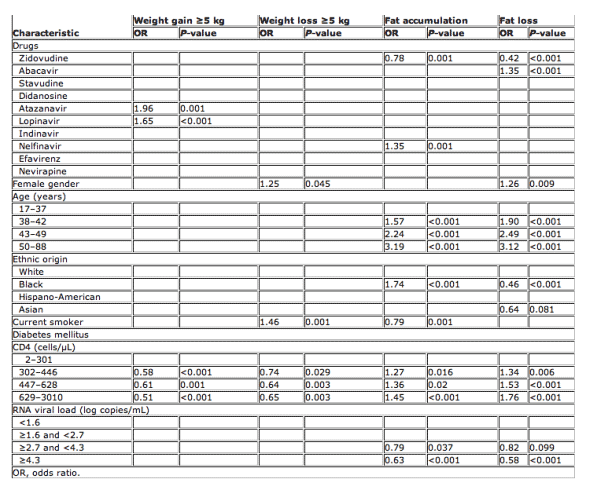
Diabetes [odds ratio (OR) 3.4, P<0.01], viral load ≥4.3 log HIV-1 RNA copies/mL (OR 2.1, P<0.001), ATV (OR 1.7, P<0.001) and LPV/r (OR 2.4, P<0.001) use, and BMI <25 and >30 (OR 1.5, P<0.001) were associated with a ≥5 kg weight gain (Table 2). Age above 37 years, a CD4 count >300 cells/μL, a plasma cholesterol value ≥5 mmol/L and the use of ABC (OR 0.7, P<0.01) were associated with a lower probability of weight gain. In multivariate analysis, ATV (OR 2, P=0.001) and LPV/r (OR 1.7, P<0.001) were associated with weight gain. A high CD4 cell count was a protective factor for weight gain (OR 0.6, P<0.001), being associated with a lower probability of weight gain (see Table 3). Patients who gained >5 kg had a mean BMI of 25.4 (after weight gain), compared with 23.6 in patients who gained <5 kg (Table 3).
Weight loss of >5 kg
A CD4 count >300 cells/μL (OR 0.7, P<0.03), a cholesterol value ≥5 mmol/L (OR 0.7, P<0.1) and EFV use (OR 0.7, P<0.03) were associated with a lower probability of weight loss ≥5 kg, while smoking (OR 1.5, P<0.001), plasma HIV RNA ≥4.3 log copies/mL (OR 1.4, P<0.02) and trigylceride values <1.75 mmol/L (OR 1.3, P<0.1) were associated with loss of weight (Table 2). In multivariate analysis, female gender (OR 1.3, P=0.04) and smoking (OR 1.5, P<0.001) were associated with increased risk of weight loss, while a high CD4 count >300 cells/μL (OR 0.7, P=0.003) was protective for weight loss (Table 3).
Fat distribution
Fat accumulation (lipohypertrophy)
Older age (OR 3, P<0.001), white ethnic origin (OR 1.3, P<0.01), diabetes (OR 3.2, P<0.01), CD4 count >300 cells/μL (OR 1.5, P<0.001), cholesterol values ≥5 mmol/L (OR 1.3, P<0.01), triglyceride values ≥1.75 mmol/L (OR 1.7, P<0.001), and ABC (OR 1.5, P<0.001), IDV (OR 1.9, P<0.001), NFV (OR 1.7, P<0.001) and EFV use (OR 1.4, P=0.001) were associated with the presence of fat accumulation. Smoking (OR 0.7, P<0.001), RNA viral load ≥4.3 log copies/mL (OR 0.5, P<0.001) and ZDV use (OR 0.8, P<0.02) were associated with less reported fat accumulation (Table 2). In multivariate analysis, older age (OR 3.2, P<0.001), black ethnic origin (OR 1.7, P<0.001), high CD4 cell count (OR 1.3, P<0.02) and NFV use (OR 1.4, P=0.001) predicted the presence of lipohypertrophy. Being a smoker (OR 0.8, P=0.02), having a high RNA viral load (>4.3 log copies/ml, P<0.001) or being on ZDV (OR 0.8, P=0.001) was associated with less fat accumulation (Table 3).
Fat loss (lipoatrophy)
Older age (OR 4.2, P<0.001), a higher CD4 cell count (OR 1.9, P<0.001), a higher cholesterol value (OR 2.1, P<0.001), a higher triglyceride value (OR 3.9, P<0.001), and ABC (OR 1.8, P<0.001), ATV (OR 1.2, P<0.03), NFV (OR 1.4, P<0.01) and EFV (OR 1.3, P<0.01) use were associated with greater lipoatrophy. BMI >25 and <30 (OR 0.8, P<0.002), black ethnicity (OR 0.3, P<0.001) and Asian ethnicity (OR 0.5, P<0.01), viral load ≥4.3 log copies/mL (OR 0.4, P<0.001), and ZDV (OR 0.5, P<0.001) and LPV (OR 0.7, P<0.04) use were associated with less atrophy (Table 2). Finally, multivariate analysis showed that lipoatrophy was associated with female gender (OR 1.3, P=0.009), older age (OR 2.5, P<0.001) high CD4 cell count (OR 1.8, P<0.001) and ABC use (OR 1.35, P<0.001). An absence of fat loss was associated with black ethnic origin (OR 0.5, P>0.001), high RNA viral load (OR 0.6, P<0.001) and use of ZDV (OR 0.4, P<0.001) (Table 3).
Table 2 Univariate analysis of factors associated with weight gain, weight loss and lipodystrophy*
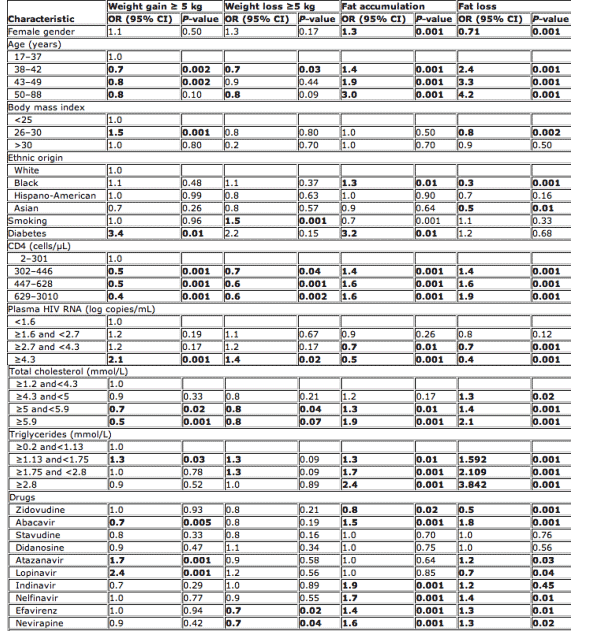
CI, confidence interval; OR, odds ratio; *values in bold indicate P-value ≦0.05.
Table 3 Multivariate analysis of factors associated with weight gain, weight loss and lipodystrophy [lipoatrophy (fat loss) and lipohypertrophy (fat gain)]
Discussion
In this study, in which more than 5500 patients were investigated within a large prospective cohort, we found that LD has become less frequent since 2003.
LD was discovered in the late 1990s, when the most frequently used drugs were d4T and indinavir. By 2006, neither was among the 10 most used antiretrovirals in Switzerland. While the use of thymidine analogues was almost universal in initial treatment in 2000, d4T is no longer recommended in the guidelines for treatment initiation [33] and had nearly disappeared by 2006, whereas ZDV tends to be replaced by TDF. Randomized prospective studies show that lipoatrophy develops less frequently on TDF than on d4T [34]. Randomized studies represent a particular situation and their results are not always reproduced in clinical practice. However, in this instance, when patients who started treatment in the 2000-2002 period were compared with those who started treatment later, we found that the probability of developing LD had decreased, while the pattern of drug prescription had significantly changed. In addition, in an analysis of all patients (not just the newly treated), the number of visits resulting in a change of treatment because of LD was found to have steadily decreased from 2003 to 2006. This indicates that, in the SHCS, the prevalence of LD serious enough to warrant a change of treatment is decreasing.
DEXA scans of patients taking standard NRTI-based cART show an early increase in limb fat before a steady and progressive limb fat loss from 3 months onwards [35]. What will happen when thymidine analogues are no longer used? One may speculate that the atrophic effect on fat tissue will disappear, but that the influence of the other constituents of cART on fat will persist. For instance, if PIs contribute to fat accumulation, their unopposed actions might lead to gain of weight.
Our results lend some support to this hypothesis, because substantial weight gain (≥5 kg within 6 months) was indeed associated with the use of the PIs LPV and ATV. By restricting our analysis to patients with a BMI >20 in men, and >18 in women, we attempted to exclude patients where AIDS-related cachexia was corrected through HAART. Nonetheless, it is still unclear whether the weight gain represents an undesirable side effect, or rather the return to desirable normalcy. As expected, the average BMI in weight gainers (25.4) was higher than the average BMI of other patients, but was still lower than the average BMI of American patients [36].
Cohort studies have inherent advantages (for instance, long follow-up and large size) but also disadvantages. Lack of randomization and masking favour bias and confounding.
Among other well-known risk factors for lipoatrophy (older age, low CD4 and high HIV RNA), we showed that ABC was associated in both univariate and multivariate analyses with a diagnosis of lipoatrophy. This was puzzling, because randomized trials demonstrated that patients randomized to ABC had greater recovery of limb fat mass compared with those remaining on thymidine analogues [37]. Examination of individual case records in the SHCS revealed that many ABC-treated patients had previously been diagnosed with lipoatrophy, leading to replacement of ZDV or d4T in patients with atrophy [37,38] by ABC; as atrophy persists for years, so does the association with ABC. In the context of this observational cohort, association does not equal causation.
Other study limitations include the lack of objective measures for fat loss and fat redistribution. However, SHCS patients are treated by specialized physicians skilled in the detection and the management of LD. Diet or lifestyle variables are not routinely recorded in the database and may limit the ability to interpret weight changes. Moreover, the database does not allow assessment of the use of ritonavir as a booster in ATV-treated patients.
In our analysis we made no distinction between current and cumulative exposure to individual ART agents. However, we have demonstrated that LD was generally diagnosed long before inclusion in our analysis in 2003.
We have scanned the medical literature and conference abstracts to search for other evidence of changing patterns and incidence of LD, but without success. The preliminary data we report here should stimulate additional research on the prevalence of LD and weight changes in large cohorts. It will be of particular interest to examine the impact of new drug classes, such as integrase or entry inhibitors, on the long-term pattern of fat redistribution and weight changes.
|
|
| |
| |
|
|
|