| |
Efficacy and Safety of Atazanavir, With or Without Ritonavir, as Part of Once-Daily HAART Regimens in ART-Naive Patients
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes:Volume 47(2)1 February 2008pp 161-167
(Niel) Malan, D. R MB, ChB*; Krantz, Edrich MB, ChB; David, Neal MB, BCh; Wirtz, Victoria MS; Hammond, Janet MD, PhD; McGrath, Donnie MD ; for the 089 Study Group
From *Triple M Research, Port Elizabeth, South Africa; Quinta-Med, Bloemfontein, South Africa; Brooklyn Medical Centre, Cape Town, South Africa; and Bristol-Myers Squibb, Research and Development, Wallingford, CT.
Several potent ritonavir (RTV)-boosted protease inhibitor (PI)-based antiretroviral (ARV) regimens are recommended as preferred agents in guidelines for the initiation of treatment of HIV disease in ARV-naive patients, including atazanavir (ATV), the first once-daily PI.1,2 RTV boosting of PIs results in improved pharmacokinetics and may improve ARV activity and decrease the risk of development of drug resistance but also increases the risk of PI-related toxicities such as dyslipidemia, fat misdistribution, and insulin resistance.1
Unboosted ATV is recommended as an alternative PI for the initiation of treatment in ARV-naive patients.1 A previous registrational study in ARV-naive patients showed that ATV (400 mg once daily) is comparable in efficacy to efavirenz, an established standard of care for initial therapy, when both are given in combination with fixed-dose zidovudine-lamivudine.3 Results from an extended use rollover/switch study demonstrated that ATV did not result in significant increases in total cholesterol, fasting low-density lipoprotein (LDL) cholesterol, non-high-density lipoprotein (HDL) cholesterol, or triglyceride levels.4 Other studies also indicated that highly active antiretroviral therapy (HAART) containing unboosted ATV5-7 may mitigate the elevations in total cholesterol, fasting LDL cholesterol, and fasting triglycerides typically associated with other PI-based HAART regimens.8-10 A randomized trial with ARV-experienced patients demonstrated that 300 mg of ATV with 100 mg of ritonavir (ATV300/RTV) resulted in better lipid profiles compared with 400 mg of lopinavir (LPV) with RTV.11 The impact of the addition of RTV to ATV-based HAART on metabolic parameters is not well characterized among ARV-naive individuals, however. Consequently, there is a need for prospective data evaluating the tradeoffs represented by a potential increase in ARV activity weighed against possible increases in toxicity attributable to RTV boosting of ATV in ARV-naive patients compared with unboosted ATV.
This study was conducted to evaluate the efficacy, safety, and tolerability of a once-daily HAART regimen of ATV, with or without RTV, in ARV-naive patients at 48 and 96 weeks. Results through 48 weeks are presented in this report.
Abstract
Background: Atazanavir (ATV), the first once-daily protease inhibitor approved for the treatment of HIV-1 infection, is recommended for use in antiretroviral (ARV) treatment-naive and -experienced patients. Study AI424-089 was a prospective, randomized, open-label, 96-week study comparing 2 ATV-based treatment regimens in ARV-naive HIV-infected patients.
Methods: Adults with HIV RNA levels ≥2000 copies/mL were randomized (1:1) to once-daily ATV at a dose of 300 mg with ritonavir at a dose of 100 mg (ATV300/RTV) or ATV at a dose of 400 mg (ATV400); both regimens included lamivudine and an investigational extended-release formulation of stavudine. The primary endpoint for this noninferiority study was the proportion of patients (response rate) with an HIV RNA load <400 copies/mL at week 48.
Results: Response rates at week 48 were 86% and 85% on the ATV300/RTV and ATV400 regimens, respectively (difference estimate [95% confidence interval] = 1.5 [-8.2 to 11.1]). There were 3 and 10 patients with virologic failure in the ATV300/RTV and ATV400 groups, respectively. One patient (ATV400) developed phenotypic resistance to ATV associated with an I50L substitution. Adverse event-related discontinuations were 8% among ATV300/RTV-treated patients and <1% among ATV400-treated patients. Plasma lipid elevations were low with both regimens. Both regimens were well tolerated.
"....The increases in total cholesterol and fasting triglycerides in this study were greater in patients receiving ATV300/RTV, suggesting that boosting with RTV may have some minimal impact on the lipid advantage of ATV; however, the changes seem to be of less magnitude than those seen with other boosted PIs..... Several findings in this study suggest that boosted ATV may be more potent than unboosted ATV in treatment-naive subjects; these include the greater TLOVR response rates at the most stringent endpoint of an HIV RNA load <50 copies/mL, the lower rates of virologic failure, the absence of major PI substitutions, and the rarity of the M184V substitution seen in subjects with virologic failure on ATV300/RTV..... unboosted ATV cannot be coadministered with the NRTI tenofovir disoproxil fumarate (DF) because of reduced levels of ATV, and RTV boosting is required in this instance.1,2 This novel head-to-head study comparing 2 different modes of ATV administration demonstrates that unboosted ATV remains a viable option for ARV-naive patients and may be of particular use for patients who are intolerant of or experience toxicity from low-dose RTV, or in areas of the world where patients do not have access to adequate refrigerated storage for RTV..."
Conclusions: These findings demonstrate the safety and efficacy of the ATV300/RTV regimen and confirm the safety and efficacy of ATV400 in an ARV-naive patient population.
RESULTS
Baseline Characteristics and Patient Disposition
A total of 200 patients were enrolled and randomized: 95 to ATV300/RTV and 105 to ATV400. Median age, gender, and racial characteristics were balanced between treatment regimens (Table 1). At baseline, patients had a median viral load of approximately 5 log10 copies/mL and a median CD4 count of approximately 200 cells/mm3 (see Table 1). Twenty-one patients discontinued study therapy before week 48 (Table 2). The most commonly reported causes of treatment discontinuations before week 48 were AEs in the ATV300/RTV group (8%) and loss to follow-up in the ATV400 group (4%).
Efficacy
Virologic response rates by TLOVR analysis (viral load <400 copies/mL) at week 48 were 86% and 85% in the ATV300/RTV and ATV400 groups, respectively; virologic response rates using the more stringent endpoint of HIV RNA level <50 copies/mL were 75% and 70%, respectively (see Table 2; Fig. 1). ATV300/RTV met the criteria for noninferiority to ATV400 in both response categories.
Virologic response was comparable between treatment regimens when stratified by qualifying HIV RNA level (Fig. 2). Among participants with qualifying HIV viral loads <100,000 copies/mL, the proportions of responders with an HIV RNA load <400 copies/mL per the TLOVR algorithm were 91% and 92% for ATV300/RTV and ATV400, respectively (87% and 82% for HIV RNA load <50 copies/mL). Among participants with qualifying HIV viral loads ≥100,000 copies/mL, 82% and 78% in the respective treatment regimens responded per TLOVR analysis at an HIV RNA load <400 copies/mL (63% and 58%, respectively, at HIV RNA load <50 copies/mL). The median CD4 cell count increase from baseline through week 48 was comparable, at 174 cells/mm3 and 213 cells/mm3 in the ATV300/RTV and ATV400 groups, respectively (see Table 2).
Genotypic and Phenotypic Resistance
Criteria for virologic failure were met by 3 ATV300/RTV-treated patients and 10 ATV400-treated patients. Paired baseline and on-study HIV samples from 2 and 8 patients, respectively, were tested successfully for phenotypic and genotypic resistance and are shown in Table 3. Four of the 8 ATV400-treated patients with virologic failure had International AIDS Society (IAS)-USA Panel-defined minor PI-related substitutions, as did 1 ATV300/RTV-treated subject.13 Major substitutions emerged in 3 ATV400-treated patients: 2 had a mixed I50I/L substitution, and the third had pure I50L and N88S substitutions. The latter patient's isolate was the only one that demonstrated phenotypic resistance to ATV; it also demonstrated increased susceptibility to saquinavir, RTV, LPV, indinavir, and amprenavir compared with the baseline isolate. No emergence of IAS-USA Panel-defined major PI substitutions was observed in ATV300/RTV-treated patients.13 Major treatment-emergent NRTI substitutions were seen in 7 patients in the ATV400 group (all M184V) and in 1 in the ATV300/RTV group (M184M/V) (see Table 3).
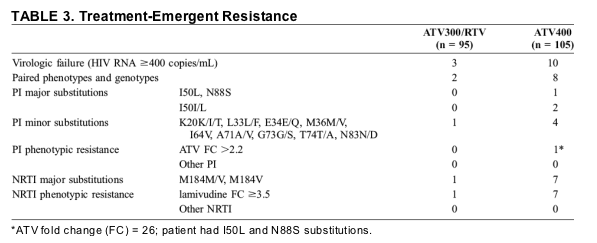
Safety
Adverse Events
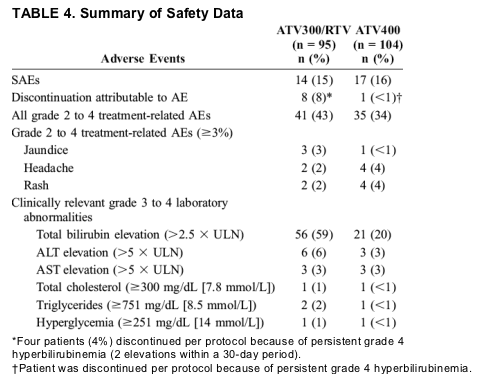
Thirty-one subjects reported at least 1 SAE: 15% in the ATV300/RTV arm and 16% in the ATV400 arm (Table 4). Overall, only 2 of 38 SAEs were considered possibly related to treatment (depression and oculogyric crisis), and both occurred in the ATV400 arm. No individual SAE was reported by more than 2 patients.
Lipids
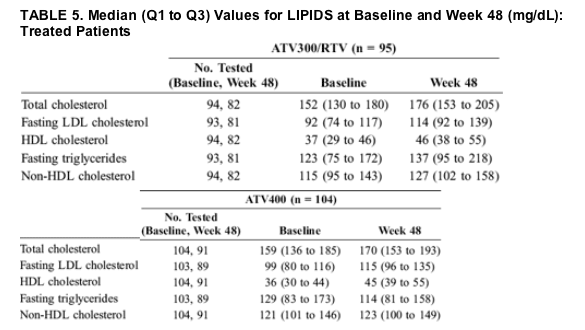
ATV300/RTV-treated patients had greater changes in all lipid parameters than did ATV400-treated patients (Table 5). Cholesterol and triglycerides were examined by National Cholesterol Education Program (NCEP) categories.15 The proportions of patients with upward shifts of at least 1 NCEP category at week 48 were comparable between the ATV300/RTV and ATV400 regimens for total cholesterol (16% and 11%) and fasting LDL cholesterol (46% and 48%), respectively. There were more patients in the ATV300/RTV group (30%) than in the ATV400 group (18%) with upward shifts of at least 1 NCEP category in fasting triglycerides. The proportions of patients with HDL cholesterol levels ≥40 mg/dL (1 mmol/L) in the ATV300/RTV and ATV400 regimens were similar at baseline (41% and 35%) and at week 48 (71% and 71%), respectively. The proportions of patients with baseline total-to-HDL cholesterol ratios >5 were 35% in the ATV300/RTV group and 38% in the ATV400 group. At week 48, the percentages decreased to 24% and 14% in the ATV300/RTV and ATV400 groups, respectively. Lipid-lowering agents were used on study by 4% of ATV300/RTV-treated patients and 3% of ATV400-treated patients.
DISCUSSION
Triple HAART containing a PI and 2 NRTIs has resulted in dramatic decreases in HIV-1-related morbidity and mortality. Three RTV-boosted PIs-ATV/RTV once daily, fosamprenavir/RTV (FPV/RTV) twice daily, and LPV/RTV twice daily-are currently recommended for the initial treatment of patients infected with HIV-1.2 FPV/RTV and LPV/RTV were compared in a large randomized controlled trial in ARV-naive patients and were found to have similar efficacy and safety through 48 weeks.10 Acknowledging the smaller size of the current study, and that there are limitations to cross-study comparisons, the response rates at the threshold of <50 copies/mL at week 48 in the current study of 75% for RTV-boosted ATV and 70% for unboosted ATV400 were comparable to those observed for FPV/RTV (66%) and LPV/RTV (65%).10
A finding of note in the current study was that response rates on both regimens varied depending on patients' qualifying HIV RNA levels. This extent of variation in response rates has not been observed in studies of other boosted PIs.10,16 For patients with viral loads ≥100,000 copies/mL on entry into the current study, the response rates at an HIV RNA level <50 copies/mL for ATV300/RTV and ATV400 were 63% and 58%, respectively. These rates are in the same range as those previously reported for LPV/RTV- and FPV/RTV-based HAART regimens among patients with baseline HIV RNA levels of ≥100,000 copies/mL or <100,000 copies/mL.10 In contrast, ATV300/RTV and ATV400 achieved greater response rates among those patients with qualifying HIV RNA levels <100,000 copies/mL, where response rates at an HIV RNA level <50 copies/mL were 87% and 82%, respectively. The mechanism for these differential response rates remains to be elucidated. A large, ongoing, international, randomized controlled trial comparing ATV300/RTV and LPV/RTV in ARV-naive patients (AI424-138) may provide further understanding of virologic response rates in all subsets of patients, including those with high baseline HIV-1 RNA levels (≥100,000 copies/mL) and those with low baseline CD4 counts (<50 cells/mm3).
Virologic failure occurred infrequently with ATV300/RTV (3 patients). This is consistent with reports of failure with other boosted PIs in ARV-naive patients from clinical trials and clinical reports.10,17-19 This further reinforces the notion that virologic failure occurs infrequently with boosted-PI-based HAART in ARV-naive patients. In contrast to ATV300/RTV, 10% of patients on ATV400 experienced virologic failure by 48 weeks. Of the 8 patients with virologic failure and typable on-study isolates, 3 had IAS-USA Panel-defined major PI substitutions. Several patients with virologic failure on ATV400 also had a detectable M184V substitution, associated with lamivudine resistance. In contrast, only 1 patient with virologic failure on ATV300/RTV developed an isolate with the mixed M184M/V substitution. The small difference in virologic failure rates and the different emergent resistance patterns likely reflect the higher ATV plasma concentrations that are achieved with RTV boosting.
Tolerability and safety and the numbers of overall treatment discontinuations were similar between the groups. The rates of grade 2 to 4 AEs and SAEs were consistent with those previously reported with ATV treatment.3 More patients on ATV300/RTV (8%) than on ATV400 (<1%) discontinued treatment because of AEs. More than half of the ATV300/RTV-treated patients who discontinued because of AEs did so by protocol mandate. These patients were required to discontinue study therapy in cases of persistent grade 4 hyperbilirubinemia irrespective of clinical symptoms. The higher rates of hyperbilirubinemia observed in the ATV300/RTV arm were likely attributable to RTV-induced increases in ATV concentrations.20 Mandated discontinuation of ATV attributable to persistent grade 4 hyperbilirubinemia does not mirror current clinical practice, in which ATV discontinuation for hyperbilirubinemia is typically recommended only when clinically evident jaundice presents a cosmetic concern to the patient. In a recent study of treatment-naive subjects treated with boosted-PI regimens, discontinuation rates at 48 weeks were 21% and 22% for FPV/RTV and LPV/RTV, respectively.10 In contrast, among treatment-naive patients receiving ATV/RTV, discontinuation rates of 12% through 48 weeks in the current study may reflect the known tolerability of ATV-based regimens in this population.3
A major concern for PI-based HAART is the development of metabolic toxicity, including dyslipidemias,21 which may contribute to increased long-term cardiovascular risk.22-24 In ARV-naive patients started on HAART, some, if not all, of the lipid changes seen in the first 48 weeks of therapy may be attributable to a return-to-health effect.25 In this study, both treatment regimens demonstrated increases from baseline in total cholesterol, fasting LDL cholesterol, and HDL cholesterol; fasting triglycerides increased from baseline only in the boosted arm. The increases in total cholesterol, fasting LDL cholesterol, and triglycerides observed in the current study were lower than those recently reported for the other boosted PIs recommended for ARV-naive patients.10 From a clinical perspective, it is important to note that few patients in this study changed NCEP categories for total cholesterol by week 48 and that the percentage of subjects with total cholesterol/HDL cholesterol ratios >5 decreased on both regimens by week 48.
The increases in total cholesterol and fasting triglycerides in this study were greater in patients receiving ATV300/RTV, suggesting that boosting with RTV may have some minimal impact on the lipid advantage of ATV; however, the changes seem to be of less magnitude than those seen with other boosted PIs.10
The increases from baseline in lipid parameters in the ATV400 arm in this study were higher than the changes seen in previous studies of unboosted ATV in ARV-naive patients.5,26 Some of the increases observed in the lipid parameters may be at least partially explained by the known impact of stavudine on lipid profiles,27,28 although the changes are greater than observed in previous studies of ATV400 used in combination with stavudine in ARV-naive subjects.26
It is important to note the limitations of this study, such as the open-label design, which may result in biased responses in safety assessments and reporting. It is unlikely, however, given the low discontinuation rate, that this study design would affect virologic, immunologic, or resistance endpoints significantly.
Several findings in this study suggest that boosted ATV may be more potent than unboosted ATV in treatment-naive subjects; these include the greater TLOVR response rates at the most stringent endpoint of an HIV RNA load <50 copies/mL, the lower rates of virologic failure, the absence of major PI substitutions, and the rarity of the M184V substitution seen in subjects with virologic failure on ATV300/RTV. As noted, however, there was not a statistically significant difference seen in any efficacy endpoint. The results do nonetheless provide useful information for clinicians to consider when selecting ATV300/RTV, LPV/RTV, and FPV/RTV, the 3 RTV-boosted PIs that are currently recommended for initial treatment of ARV-naive patients.1,2
Factors that affect selection of first-line ARV therapy are many, and include efficacy, tolerability, the need to preserve future treatment options, and, in some areas of the world, the availability of refrigeration for the storage of RTV. In addition, drug-drug interactions may have an impact on the choice of agents used in first-line ARV therapy. For instance, unboosted ATV cannot be coadministered with the NRTI tenofovir disoproxil fumarate (DF) because of reduced levels of ATV, and RTV boosting is required in this instance.1,2 This novel head-to-head study comparing 2 different modes of ATV administration demonstrates that unboosted ATV remains a viable option for ARV-naive patients and may be of particular use for patients who are intolerant of or experience toxicity from low-dose RTV, or in areas of the world where patients do not have access to adequate refrigerated storage for RTV. The unique resistance profile of ATV also may preserve the option of future treatment with other PIs.
In conclusion, these findings demonstrate the safety and efficacy of the ATV300/RTV regimen and confirm the safety and efficacy of ATV400 in an ARV-naive patient population. Further studies to assess the safety, efficacy, and metabolic effects of ATV300/RTV compared with a second standard-of-care boosted PI should better characterize the use of ATV with RTV in HIV-infected ARV-naive patients.
METHODS
Study Population
The study was conducted at 30 centers in 10 countries in North and South America, Africa, and Europe. HIV-infected adult men and women with a screening plasma HIV RNA level ≥2000 copies/mL (no CD4 cell count restrictions) were eligible for enrollment. Patients were excluded if they had previously received >30 days of nucleoside reverse transcriptase inhibitor (NRTI) therapy, >7 days of nonnucleoside reverse transcriptase inhibitor (NNRTI) or PI therapy, or any ARV therapy within 30 days of screening. Patients were randomized to once-daily treatment with ATV300/RTV or ATV at a dose of 400 mg (ATV400). Both groups also received 300 mg of lamivudine once daily and an investigational extended-release formulation of stavudine once daily (dosage adjusted for patient weight). Extended-release stavudine is not commercially available.
The study was approved by institutional review/ethics boards at all participating centers, and each participant provided signed informed consent before enrollment in the study.
Study Design and Procedures
This was a randomized, open-label, 2-arm study designed to demonstrate noninferiority of ATV300/RTV to ATV400. The primary endpoint was a comparison of the proportion of patients with an HIV RNA load <400 copies/mL at 48 weeks.
Participants were assessed at baseline and at weeks 2, 4, 8, 12, 16, 24, 32, 40, and 48. At each visit, participants were evaluated for toxicities and serum was collected for immunologic, virologic, resistance, chemistry, and hematology testing.
HIV RNA was measured using the AMPLICOR HIV-1 MONITOR test, version 1.5 (Roche Diagnostics, Pleasanton, CA). HIV isolates were tested for phenotypic resistance using the PhenoSense assay (Monogram Biosciences, South San Francisco, CA); substitutions to HIV reverse transcriptase and protease genomes were determined using the GenoSure assay (LabCorp of America, Burlington, NC). Resistance data for virologic failures were generated in real time and were made available to investigators for assistance in subsequent treatment decisions.
Safety assessments included reported adverse events (AEs); serious AEs (SAEs); discontinuations attributable to AEs; measured laboratory abnormalities; and changes from baseline in total cholesterol, fasting triglycerides, fasting LDL cholesterol, and HDL cholesterol.
Statistical Analysis
A sample size of 200 patients (100 per group) provided at least 80% power to demonstrate noninferiority of ATV300/RTV to ATV400 as measured by the proportion of patients with an HIV RNA load <400 copies/mL at week 48. Noninferiority was considered to have occurred if the lower 95% confidence limit for the difference in proportions of responders in the ATV 300/RTV group versus ATV400 group was >-10%. The primary analysis was based on randomized intent-to-treat (ITT) patients using a time-to-loss-of-virologic-response (TLOVR) algorithm.12 Randomization was stratified by qualifying HIV RNA viral load (<100,000 copies/mL or ≥100,000 copies/mL). Identical statistical methods were used for the secondary endpoint (HIV RNA load <50 copies/mL). Treatment-emergent genotypic and phenotypic resistance profiles were assessed for patients with virologic failure through week 48.
The frequency of AEs, SAEs, death, laboratory abnormalities, and discontinuations attributable to AEs, and absolute and mean percent changes from baseline in serum lipids, were summarized through week 48. Differences in lipids between the groups were compared using 95% confidence intervals based on a t test stratified by qualifying HIV RNA. Data obtained after initiation of lipid-lowering therapy were excluded from all lipid analyses.
|
|
| |
| |
|
|
|