| |
Nevirapine (HSR) Hypersenstivity Reaction Safety & CD4 & Viral Load
|
| |
| |
Discontinuation of Nevirapine Because of Hypersensitivity Reactions in Patients with Prior Treatment Experience, Compared with Treatment-Naive Patients: The ATHENA Cohort Study
Clinical Infectious Diseases March 15, 2008;46:933-940
Ferdinand W. N. M. Wit,1,a Anouk M. Kesselring,2,a Luuk Gras,2 Clemens Richter,4 Marchina E. van der Ende,5 Kees Brinkman,3 Joep M. A. Lange,1 Frank de Wolf,2 and Peter Reiss1
1Center for Infection and Immunity Amsterdam, Center for Poverty-Related Communicable Diseases, and Department of Infectious Diseases, Tropical Medicine and AIDS, Academic Medical Center, University of Amsterdam, 2HIV Monitoring Foundation, and 3Onze Lieve Vrouwe Gasthuis, Amsterdam, 4Rijnstate Hospital, Arnhem, and 5Erasmus Medical Center, Rotterdam, The Netherlands
"We conclude that, compared with treatment-naive patients with low CD4 cell counts, treatment-experienced patients with a low pretreatment CD4 cell count and a high current CD4 cell count are at increased risk of developing a treatment-limiting HSR after starting NVP therapy. However, this group of patients effectively consists of 2 clinically distinct subpopulations: (1) patients with an undetectable viral load who switch to NVP therapy because of toxicity or for simplification of otherwise successful therapy and (2) patients with a detectable viral load who initiate NVP therapy as part of salvage therapy. Patients with an undetectable viral load at the initiation of NVP therapy do not have an elevated risk of developing an HSR, compared with treatment-naive patients with low CD4 cell counts. However, treatment-experienced patients with a detectable viral load indeed have a significantly increased risk of developing an HSR. Therefore, physicians should continue to adhere to current treatment guidelines for treatment-experienced patients with detectable viremia and should avoid initiating NVP therapy in this group whenever possible. In contrast, if our results are independently confirmed by other research groups, physicians may consider prescribing NVP to treatment-experienced patients with a low nadir CD4 cell count, a high current CD4 cell count, and an undetectable plasma viral load. Future studies are needed to determine whether this would apply equally to both women and men of different ethnicities."
ABSTRACT
Background. Recommendations that nevirapine (NVP) should be avoided in female individuals with CD4 cell counts >250 cells/μL and in male individuals with CD4 cell counts >400 cells/μL are based on findings in treatment-naive patients. It is unclear whether these guidelines also apply to treatment-experienced patients switching to NVP-based combination therapy.
Methods. Patients in the ATHENA cohort study who had used NVP-based combination therapy were included. We identified patients who discontinued NVP-based combination therapy because of hypersensitivity reactions (HSRs; rash and/or hepatotoxicity) within 18 weeks after starting such therapy. We grouped patients according to their CD4 cell count at the start of NVP-based combination therapy (current CD4 cell count) as having a high CD4 cell count (for female patients, >250 cells/μL; for male patients, >400 cells/μL) or a low CD4 cell count. Treatment-experienced patients were further subdivided according to the last available CD4 cell count before first receipt of antiretroviral therapy (ART; pre-ART CD4 cell count) using the same criteria. Risk factors for HSR were assessed using multivariate logistic regression.
Results.
Of 3752 patients receiving NVP-based combination therapy, 231 patients (6.2%) discontinued NVP therapy because of HSRs.
Independent risk factors included female sex and Asian ethnicity.
Having an undetectable viral load (VL) at the start of NVP therapy was associated with reduced risk of developing an HSR (adjusted odds ratio [OR], 0.52; 95% confidence interval [CI], 0.38-0.71).
Pretreated patients with low pre-ART and high current CD4 cell counts and a detectable VL when switching to NVP-based combination therapy had a significantly higher risk of developing an HSR, compared with treatment-naive patients who started NVP therapy with low CD4 cell counts (adjusted OR, 1.87; 95% CI, 1.11-3.12); pretreated patients with low pre-ART CD4 cell counts who switched to NVP therapy with a high current CD4 cell count and an undetectable VL did not have an increased risk of developing an HSR (adjusted OR, 1.03; 95% CI, 0.66-1.61).
Conclusions. Treatment-experienced patients who start NVP-based combination therapy with low pre-ART and high current CD4 cell counts and an undetectable VL have a similar likelihood for discontinuing NVP therapy because of HSRs, compared with treatment-naive patients with low CD4 cell counts. This suggests that NVP-based combination therapy may be safely initiated in such patients. However, in similar patients with a detectable VL, it is prudent to continue to adhere to current CD4 cell count thresholds.
Rash and hepatotoxicity are the most frequent toxicities associated with nevirapine (NVP). Six percent to 7% of patients starting NVP therapy need to stop NVP therapy because of such events [1-4]. Life-threatening rashes occur in <1% of patients [5-7]. NVP-associated symptomatic hepatitis resulting in hepatic failure is also rare but, likewise, potentially lethal [8, 9]. Most of these reactions, which are thought to be indicative of a hypersensitivity reaction (HSR), occur within the first 18 weeks after starting NVP therapy. Women with high CD4 cell counts have an increased risk of developing severe HSRs [10]. The NVP package insert has recently been amended with a black-box warning to avoid starting NVP therapy in female patients with CD4 cell counts >250 cells/μL and in male patients with CD4 cell counts >400 cells/μL, unless the benefits outweigh the risks. These recommendations are based largely on findings in treatment-naive patients. It is unknown whether the same CD4 cell count thresholds apply to pretreated patients who may have experienced substantial CD4 cell count increases during prior treatment.
Methods
All 22 Dutch hospitals providing antiretroviral treatment (ART) participate in the AIDS Therapy Evaluation in The Netherlands (ATHENA) cohort study, and all patients have provided written informed consent for their data to be anonymously recorded in a central database that is maintained by the HIV Monitoring Foundation [11].
We selected all patients who had started NVP therapy from April 1998 (when NVP became available in The Netherlands) through July 2006 (we used the 2006 version of the ATHENA database for this analysis) and for whom data were available on their last known CD4 cell count before their first use of any ART (i.e., nadir CD4 cell count) and before initiation of NVP therapy. Only CD4 cell counts measured within 24 weeks before these points were taken into account. We identified all patients who discontinued NVP therapy because of toxicities compatible with a NVP-associated HSR within 18 weeks after starting NVP therapy. All rashes and/or hepatotoxicity that occurred within 18 weeks after initiation of therapy and that led to discontinuation of NVP therapy were scored as NVP-associated HSRs. All reasons for discontinuation have been systematically collected and validated in the ATHENA database. Clinical management of rashes and hepatotoxicity was performed according to guidelines issued by Boehringer Ingelheim (in the Viramune package insert). If additional antiretroviral drugs were stopped at the same time that NVP was stopped, reasons for discontinuation of these drugs were also considered for NVP.
Patients were classified into 2 groups: ART-naive patients starting NVP therapy and ART-experienced patients starting NVP therapy. Both ART-naive and -experienced patients were grouped on the basis of having a high CD4 cell count (for female patients, >250 cells/μL; for male patients, >400 cells/μL) or a low CD4 cell count at the start of NVP therapy. The treatment-experienced patients were further subdivided on the basis of the last available pretreatment CD4 cell count (nadir CD4 cell count) being high or low, using the same criteria.
The proportions of patients discontinuing NVP therapy because of HSRs were compared between groups with use of the ƒÔ2 test. The influence of the following other factors possibly associated with NVP-associated HSR were investigated using logistic regression: sex, country of origin (as surrogate for ethnicity), age, prior diagnosis of AIDS, hepatitis B and C virus coinfections, body mass index (calculated as the weight in kilograms divided by the square of the height in meters), previous treatment with any antiretroviral agent, prior treatment with efavirenz, and having an undetectable viral load. Parameters identified by univariate analysis as significantly associated with developing an HSR were entered into a multivariate logistic regression model with use of stepwise selection of variables.
Mortality rates during the first 24 weeks after starting NVP-based combination ART were compared between patients who experienced HSRs and those who did not experience HSRs. All analyses were performed using SAS, version 9 (SAS Institute).
Results
At the time of analysis, the ATHENA database contained information regarding ART for 11,592 HIV-infected patients. Of these patients, 3752 had used NVP and had CD4 cell counts available both at the start of NVP treatment and, in the case of patients with prior treatment experience, shortly before the start of their first receipt of ART. Characteristics for treatment-naive and -experienced patients who did or did not experience HSRs are summarized in table 1. NVP was discontinued because of an HSR in 231 patients (6.2%). The HSR consisted of rash in 182 patients (4.9%) and hepatotoxicity in 57 patients (1.5%; in 8 patients, rash and hepatotoxicity occurred simultaneously). As expected, there were substantial differences between the patients who had to stop therapy because of an HSR and those who did not. HSRs occurred more often in women (p<.001), patients with higher CD4 cell counts (p<.001), patients who had not received a prior diagnosis of AIDS (p=.01), and pretreated patients with a detectable viral load at the start of NVP therapy (p=.008).
A total of 934 patients (24.9%) were treatment naive at the time they started receiving NVP therapy; 2818 patients (75.1%) were ART experienced. Of 934 treatment-naive patients, 719 (77.0%) had a low CD4 cell count and 215 (23.0%) had a high CD4 cell count at initiation of NVP therapy. Of the 2818 treatment-experienced patients, 1366 (48.5%) had a low pre-ART CD4 cell count and a low CD4 cell count at initiation of NVP therapy, 852 (30.2%) had a low pre-ART CD4 cell count but had a high CD4 cell count at initiation of NVP therapy, and 483 (17.1%) had a high pre-ART CD4 cell count and a high CD4 cell count at initiation of NVP therapy. Only a small group of patients (117 patients [4.2%]) had a high pre-ART CD4 cell count but a low CD4 cell count at initiation of NVP therapy.
Figure 1 shows the proportions of patients who discontinued NVP therapy because of an HSR in each of the 6 groups. Treatment-naive patients with low CD4 cell counts discontinued NVP therapy at a frequency similar to that for pretreated patients with low pre-ART and low current CD4 cell counts (5.2% vs. 4.4%; p=.44).
Figure 1. Risk of developing an nevirapine-associated hypersensitivity reaction (HSR), with patients stratified according to their CD4 cell count at the first use of antiretroviral therapy (ART; nadir CD4 cell count) and their CD4 cell count at the start of nevirapine therapy (current CD4 cell count). High CD4 cell counts were defined as CD4 cell counts >250 cells/μL in female patients and CD4 cell counts >400 cells/μL in male patients. Data are for 719 and 215 ART-naive patients with low and high CD4 cell counts, respectively. Among ART-experienced patients, 1366 had low nadir and low current CD4 cell counts, 852 had low nadir and high current CD4 cell counts, 117 had high nadir and low current CD4 cell counts, and 483 had high nadir and high current CD4 cell counts.
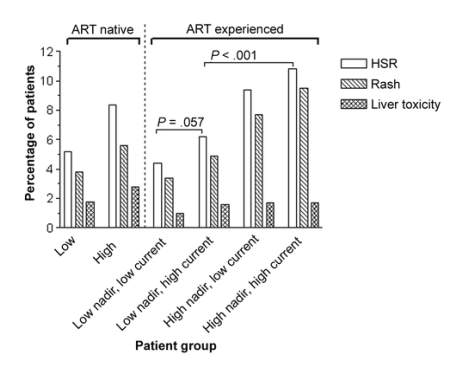
Similarly, HSRs were observed at a similar frequency in treatment-naive patients starting NVP therapy with a high CD4 cell count and in pretreated patients with high pre-ART and high current CD4 cell counts (8.4% vs. 10.8%; p=.33). HSRs occurred more frequently in pretreated patients with a low pre-ART CD4 cell count and a high current CD4 count than in pretreated patients with low pre-ART and current CD4 counts (6.2% vs. 4.4%; p=.057) but occurred significantly more frequently in pretreated patients with high pre-ART and high current CD4 cell counts than in the former patient group (10.8% vs. 6.2%; p=.003).
Univariate and multivariate logistic regression analyses were performed to investigate the influence of several parameters on the odds of discontinuing NVP therapy because of an HSR. After stepwise selection, the remaining significant factors in the multivariate model were presented in the final model. Parameters identified by univariate analysis as statistically significantly associated with the odds of developing an HSR were female sex (p<.001), country of origin (Asian country, p=.02), prior diagnosis of AIDS (), and having a detectable viral load (p=.007). The effects of age, hepatitis B or C virus coinfection, body mass index, and other components of the ART regimens were also investigated, but none of these variables had statistical significance in univariate models.
The final multivariate model is presented in table 2. This analysis confirmed that, compared with treatment-naive patients with low CD4 cell counts, pretreated patients with low pre-ART and high current CD4 counts have a statistically significant increased risk of having to discontinue NVP therapy because of an HSR. Other independent risk factors were female sex, Asian ethnicity, and having a detectable viral load at the start of NVP therapy. There was a statistically significant interaction between female sex and Asian ethnicity, with Asian women but not Asian men having a markedly increased risk of developing an NVP-associated HSR. Women, in general, had a 39% higher risk of developing an HSR, compared with men, and the risk of Asian women developing an HSR was even higher (11 of 40 Asian women discontinued NVP therapy because of an HSR).
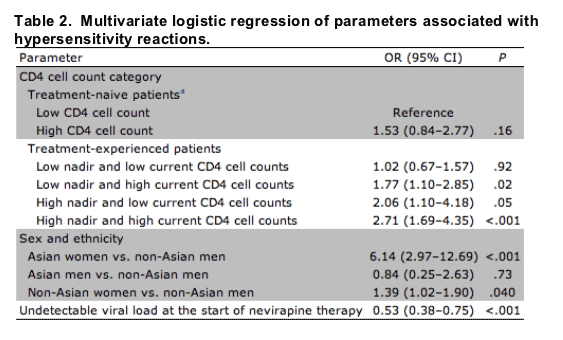
NOTE. The nadir CD4 cell count was the CD4 cell count at the start of first antiretroviral therapy, and current CD4 cell count was the CD4 cell count at the start of nevirapine therapy in pretreated patients. High CD4 cell count was defined as a CD4 cell count >250 cells/μL in female patients and a CD4 cell count >400 cells/μL in male patients.
aThe reference is the group of treatment-naive patients with low CD4 cell count at the start of nevirapine therapy.
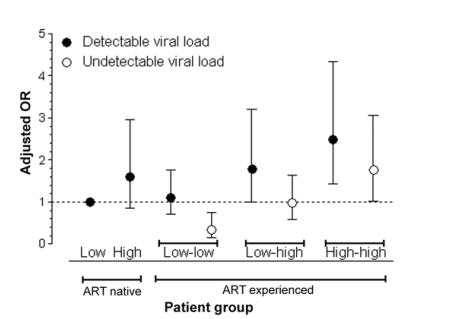
The effect of having an undetectable viral load on the risk of developing an HSR was statistically and clinically highly significant; the risk almost doubled among patients with a detectable viral load. Adjusting for sex and country of origin, we calculated ORs for each patient category subdivided according to patient viral load at the start of NVP therapy (figure 2). The unadjusted rates of HSR in the patient groups were 5.3% (33 of 629 of the treatment-naive patients with a low CD4 cell count), 8.9% (17 of 190 of the treatment-naive patients with a high CD4 cell count), 12.4% (28 of 225 of the treatment-experienced patients with a detectable viral load and high pre-ART and current CD4 cell counts), 9.3% (24 of 258 of the treatment-experienced patients with an undetectable viral load and high pre-ART and current CD4 cell counts), 5.7% (46 of 806 of the treatment-experienced patients with a detectable viral load and low pre-ART and current CD4 cell counts), 1.7% (8 of 463 of the treatment-experienced patients with an undetectable viral load and low pre-ART and current CD4 cell counts), 9.3% (20 of 215 of the treatment-experienced patients with a detectable viral load and low pre-ART and high current CD4 cell counts), and 5.2% (31 of 596 of the treatment-experienced patients with an undetectable viral load and low pre-ART and high current CD4 cell counts). We investigated whether having low-level detectable viremia was associated with an increased risk of discontinuation of NVP therapy because of an HSR. However, because of the low number of patients, the analysis did not reach statistical significance. There was a sharp increase in the risk of discontinuation of NVP therapy because of an HSR in patients with low-level viremia. Compared with the patients with an undetectable viral load, the 184 patients with low-level viremia (defined as a detectable viral load <400 copies/mL) had an increased risk of discontinuation of NVP therapy because of an HSR (adjusted OR, 1.75; 95% CI, 0.95-3.21).
Figure 2. Risk of developing an nevirapine (NVP)-associated hypersensitivity reaction, with patients stratified according to their CD4 cell count and plasma viral load at the start of NVP therapy. Error bars represent 95% CIs. The reference group is treatment-naive patients with a detectable plasma viral load. Analysis is adjusted for sex and country of origin. Black circles represent detectable viral load (defined as an HIV RNA load >50 copies/mL) at the start of NVP therapy. White circles represent undetectable viral load at the start of NVP therapy. "Low" and "high" antiretroviral therapy (ART)-naive groups had low and high CD4 cell counts at the start of NVP therapy, respectively. "Low-low" ART-experienced patients had a low nadir CD4 cell count and a low CD4 cell count at the start of NVP therapy, "low-high" patients had a low nadir CD4 cell count and a high CD4 cell count at the start of NVP therapy, and "high-high" patients had a high nadir CD4 cell count and a high CD4 cell count at the start of NVP therapy.
To test the hypothesis that prior treatment with efavirenz might lower the risk of developing an HSR by inducing hepatic cytochrome p450 isoenzymes that are involved in the metabolization of NVP, we repeated the multivariate logistic regression analyses including only the pretreated patients, 226 of whom substituted NVP for efavirenz. After adjusting for the above-described parameters significantly associated with the risk of HSR, prior treatment with efavirenz was not found to be a risk factor for development of an HSR (adjusted OR, 1.0; ).
We compared mortality rates during the first 24 weeks after starting NVP-based combination ART. We found no statistically significant differences in mortality rates between the patients who experienced an NVP-associated HSR and those who tolerated NVP (0.9% vs. 1.1%, p=1.0), between patients who experienced NVP-associated rashes and those who did not (1.1% vs. 1.1%; p=1.0), or between those who experienced NVP-associated hepatotoxicities and those who did not (0% vs. 1.1%; p=1.0). Only 2 patients who experienced an NVP-associated HSR died within 24 weeks after starting NVP therapy. Both of these deaths were unrelated to the use of NVP (1 sudden death [no autopsy performed] and 1 traffic accident).
The majority of the NVP-associated HSRs consisted of rashes with or without concomitant liver enzyme elevations, and only a minority of HSRs (n=57) were isolated liver-related events (figure 1). There was not enough power to investigate whether the same risk factors apply for these isolated liver-related events. We repeated the final multivariate model using only rash as an end point and found no significantly different results.
Discussion
As expected, treatment-experienced patients with a low nadir CD4 cell count and a low CD4 cell count at the start of NVP therapy had a risk of having to discontinue NVP therapy because of an HSR that was similar to the risk for treatment-naive patients with low CD4 cell counts. The same was true for treatment-experienced patients with a high nadir CD4 cell count and a high current CD4 cell count when compared with treatment-naive patients with high CD4 cell counts. The proportion of treatment discontinuations among treatment-experienced patients with a low nadir CD4 cell count but a high current CD4 count was between the proportions among the aforementioned treatment-experienced groups who had low nadir and current CD4 cell counts or high nadir and current CD4 cell counts. In the multivariate logistic regression analysis, we found that the risk of developing an HSR was positively associated with higher CD4 cell count, female sex (especially women of Asian ethnicity), and having a detectable viral load at the start of NVP therapy. We found that treatment-experienced patients with a low nadir CD4 cell count but a high current CD4 cell count who had undetectable viral loads at the start of NVP therapy had a lower risk of developing HSRs that was comparable to that for treatment-naive patients with a low CD4 cell count. However, the treatment-experienced patients with a low nadir CD4 cell count, a high current CD4 cell count, and a detectable viral load had a higher risk of developing an HSR that was comparable to that for treatment-naive patients with a high CD4 cell count.
We previously reported that patients starting NVP therapy with an undetectable viral load appeared to have a low incidence of HSR [12, 13]. The current analysis corroborates these findings. A possible explanation is that, when HIV replication is controlled, the lower antigenic HIV load results in less immune hyperstimulation, which, in turn, might reduce the tendency of the immune system to overreact to the institution of NVP treatment. This hypothesis, however, does not explain why HIV-seronegative adults, who would not be expected to have hyperstimulated immune systems, are prone to developing life-threatening HSRs while receiving NVP therapy [5].
Our findings are in accordance with the meta-analysis performed by other authors (E. De Lazzari, A. Leon, and J. A. Arnaiz, E. Martinez, J. Mallolas, J. L. Blanco, M. Laguno, M. Larrousse, A. Milinkovic, M. Lonca, and J. M. Gatell, unpublished data), who found no evidence of an increased risk of HSR in treatment-experienced patients who had an undetectable viral load at the start of NVP therapy. Those authors did not find an influence of Asian ethnicity on the incidence of HSR; however, their data set contained very few Asian patients (only 18 of 2545 total patients). Mocroft et al. [14] used the EuroSIDA database to study discontinuations of NVP therapy because of toxicity or physician and/or patient choice in treatment-naive and -experienced patients. Likewise, they reported a significantly lower rate of discontinuation of NVP therapy in the 3 months after the start of NVP therapy in treatment-experienced patients with a high CD4 cell count, compared with the reference group of treatment-naive patients with high CD4 cell counts. However, their toxicity end point consisted of all toxicities caused by the regimen that the patients received and was not limited to events that were likely to represent NVP-associated HSRs. Furthermore, in the EuroSIDA data set, there was only a small number of patients with a high CD4 cell count in the treatment-naive group.
Shenton et al. [15] found that CD4 cells were the likely mediators of an immune-mediated mechanism for an idiosyncratic NVP-associated HSR in an animal model of the female brown Norway rat. Their results provide an explanation for the observation that NVP-associated HSRs occur more frequently in both HIV-seronegative persons [5, 16-19] and in HIV-infected patients with higher CD4 cell counts. Additional evidence that NVP-associated HSRs are immune mediated was provided by Martin et al. [20], who found that HLA-DRB1*0101 predisposes to HSRs. The observation that HIV-exposed but uninfected infants who are born to HIV-infected mothers and who receive NVP treatment for several months during the breast-feeding period rarely, if ever, develop HSRs might be explained by these neonates still having immature immune systems that are incapable of mediating such a response [21].
Although, in the univariate analyses, a higher body mass index was associated with an increased risk of developing an HSR, we did not find this association in the multivariate model. This univariate association may be explained by the fact that pregnant women were also included in our analysis and that HSRs are known to occur more frequently during pregnancy [22]. Of 125 pregnant women in our data set, 14 developed HSRs (6 experienced hepatotoxicity, 7 experienced rash, and 1 experienced both). In addition, we did not find evidence that induction of cytochrome p450 enzymes through the prior use of efavirenz lowers the risk of developing an HSR. These findings suggest that peak NVP concentrations are not associated with a higher risk of developing an HSR. The 2NN study found that NVP plasma concentrations were not associated with the occurrence of clinical hepatic events, elevations in asymptomatic liver enzyme levels, or rash [23], although a once-daily dose of NVP resulted in higher rates of HSR, compared with a twice-daily dose of NVP [24, 25]. The 2NN study confirmed that higher CD4 cell count, female sex, and geographical region (Asia; probably being a proxy for ethnicity) were correlated with the likelihood of experiencing an NVP-associated HSR [26]. Other researchers have also reported higher rates of HSR in women [10, 27] and Asian persons [28]. Women also appear to be at increased risk of developing HSR while receiving an antiretroviral agent other than NVP [29]. In our data set, we found that Asian women in particular have a statistically significant increased risk of developing an HSR. Asian men did not have an increased risk of developing an HSR, compared with white men.
This study has several limitations that are mostly inherent to retrospective observational cohort studies in general. Data regarding liver enzyme levels were collected only when the levels were clinically significantly elevated. It was not possible to ascertain whether physicians who attributed discontinuations of NVP therapy to hepatotoxicity fully adhered to the guidelines in the NVP package insert, although these guidelines are commonly adhered to in The Netherlands. Low-grade rashes that did not result in modification of treatment might not always have been captured in the clinical records; thus, the total number of all-grade rashes could not reliably be determined. The group of patients of Asian ethnicity constituted only a small minority of the total population. Although the overall sample size of our study was large, the 95% CIs in the final multivariate model were quite wide because of the considerable number of subgroups analyzed.
Our results are relevant for ART programs in developing countries. Implementation of combination ART in resource-limited regions is currently still being prioritized mainly toward patients with advanced immunodeficiency (CD4 cell count, <200 cells/mm3) and/or symptomatic HIV disease, but this approach, unfortunately, is associated with high rates of early mortality [30]. Hopefully, as more resources become available in developing countries, it can be expected that recommendations regarding when to initiate combination ART in such countries will focus on persons with higher CD4 cell counts. As long as NVP remains an important component of initial combination ART in such countries, higher rates of potentially life-threatening NVP-associated HSR, particularly among women, are likely.
We conclude that, compared with treatment-naive patients with low CD4 cell counts, treatment-experienced patients with a low pretreatment CD4 cell count and a high current CD4 cell count are at increased risk of developing a treatment-limiting HSR after starting NVP therapy. However, this group of patients effectively consists of 2 clinically distinct subpopulations: (1) patients with an undetectable viral load who switch to NVP therapy because of toxicity or for simplification of otherwise successful therapy and (2) patients with a detectable viral load who initiate NVP therapy as part of salvage therapy. Patients with an undetectable viral load at the initiation of NVP therapy do not have an elevated risk of developing an HSR, compared with treatment-naive patients with low CD4 cell counts. However, treatment-experienced patients with a detectable viral load indeed have a significantly increased risk of developing an HSR. Therefore, physicians should continue to adhere to current treatment guidelines for treatment-experienced patients with detectable viremia and should avoid initiating NVP therapy in this group whenever possible. In contrast, if our results are independently confirmed by other research groups, physicians may consider prescribing NVP to treatment-experienced patients with a low nadir CD4 cell count, a high current CD4 cell count, and an undetectable plasma viral load. Future studies are needed to determine whether this would apply equally to both women and men of different ethnicities.
|
|
| |
| |
|
|
|