| |
TILT: a randomized controlled trial of interruption of antiretroviral therapy with or without interleukin-2 in HIV-1 infected individuals
|
| |
| |
AIDS:Volume 22(6)30 March 2008p 737-740
From Jules Levin: 86 Patients, median CD4 of 750, nadir CD4 of 268, all had <200 c/ml & 88% <50 c/ml. Patients had been taking a median of six antiretroviral drugs for a median of 5 years. There were 3 study arms: continued ART, interruption, and interruption after IL-2 therapy. The study results found that use of IL-2 increased CD4 count high before interruption: at interruption the CD4 count in the IL-2 arm was a median of 1800 (range 683-4800). Patients in the IL-2 are remained off HAART much longer, only 34% had restarted HAART after 2 years. But during interruption CD4 counts declined to 268 and for some patients to 100 or less during interruption when on IL-2, and viral load was detectable: 10,000 copies/ml and higher, a median of 4.2 log (2.9-4.6 log). At CROI 2008 several studies reported problems associated with low CD4 and detectable viral load. Two studies reported elevated inflammation markers associated with detectable viral load; one from the SMART study, the author saying that cardiovascular risk and other non-AIDS events can be associated with detectable viral load. Two additional studies found low CD4 count and viral load associated with cancers. A study from Hopkins found just being on ART reduced the risk for developing diseases, both infectious and non-infectious, for patients in the same CD4 category, even if CD4 count was above 350. Another study found that even after resuming therapy in SMART that did not eliminate the risk for developing risk events: Resuming antiretroviral therapy for over 2 years in the SMART trial did not erase the higher risk of progression to AIDS, non-AIDS diseases, or death in the interruption group.
AIDS:Volume 22(6)30 March 2008p 737-740
[CLINICAL SCIENCE]
Angus, Briana,b; Lampe, Fionac; Tambussi, Guiseppef; Duvivier, Claudineg; Katlama, Christineg; Youle, Mikec; Williams, Iand; Clotet, Bonaventurah; Fisher, Martine; Post, Frank Ad; Babiker, Abdulb; Phillips, Andrewc; TILT trial Steering Committee
From the aNuffield Department of Medicine, John Radcliffe Hospital, Oxford, UK
bMedical Research Council, London, UK
cRoyal Free and University College London Medical School, UK
d5 King's College London, London, UK
eRoyal Sussex County Hosp, Brighton, UK
fMario Negri Inst, Milan, Italy
gHosp Pitie-Salpetriere, Paris, France
hUniv Hosp Germans Trias i Pujol, Barcelona, Spain.
"SMART and other trials suggest that treatment interruption should be discouraged strongly [6,7]. However, patients continue to express the wish to interrupt therapy against such advice [11] and, while the clinical benefit associated with CD4 cell count rises with IL-2 awaits evaluation [12], use of IL-2 may be considered in some cases to help to maintain a higher CD4 cell count......Compared with patients who interrupting treatment without IL-2, there was a significantly longer time to restarting ART in those who received IL-2. A starting dose of IL-2 4.5 MIU twice daily for 5 days with concomitant ART was well tolerated with little need for dose reduction..... There were 39 grade 3 and 4 adverse events, with 21 in arm C:.... Therapy was restarted after interruption in 20 subjects in arm B and 18 in arm C. Of these, 19 and 16, respectively, had follow-up at 24 weeks; 94% in arm B and 91% in arm C had viral load < 200 copies/ml..... By week 105, there was a 66% probability of having restarted ART in arm B compared with 34% in arm C (log rank P = 0.002)..... Median viral load values were higher from week 9 in the two interruption arms. Arm C had higher viral load levels than B, presumably related to the greater proportion not taking ART at the time. At week 105, there was a highly significant difference between arms: 1.7 log copies/ml, (IQR, 1.7-2.3), 2.3 log copies/ml (IQR, 1.7-4.4) and 4.2 log copies/ml (IQR, 2.9-4.6) in arms A, B and C, respectively (Kruskall-Wallis P < 0.0001), with the interruption arms having much higher levels than the continuation arm..... In arm C, 31 patients interrupted therapy at week 9 (IQR 8-25) The median CD4 cell count at ART interruption was 1810 cells/μl (IQR, 683-4817), and ART interruption lasted a median of 96 weeks.... Of the 27 patients in arm A, nine interrupted therapy for a median of 22 weeks (IQR, 2-74) by patient wish and two remained off ART during the study period. Of the 28 patients in arm B, 27 interrupted therapy (one withdrew consent). The interruption occurred at a median of week 9 (IQR, 8-14) as per protocol and the median CD4 cell count at interruption was 694 cells/μl (IQR, 199-1247). The initial interruption lasted a median of 50 weeks...."
Abstract
Objective: We aimed to see if structured treatment interruption (STI) could be supported safely with the use of two cycles of IL-2 (4.5 MIU q12h subcutaneously 5 days) before STI to prolong the time before therapy restarted.
Methods: Subjects were randomly allocated to either A - continuous ART; B - continue for 9 weeks, then STI; restart with the same ART when the CD4 count falls below 200 cells; or C - two cycles of IL-2, 8 weeks apart, while still on ART; at week 9 stop ART and use a new cycle of IL-2 alone whenever the CD4 count falls < 300 cells. Patients were followed until week 105.
Results: 86 mostly white middle aged homosexual men with a baseline median CD4 count of 754 cells/ml (range 240-1400) and a nadir CD4 count of 268 cells/ml (range 62-822) enrolled. By 96 weeks there was a 66% probability of having restarted ART in arm B compared with 34% in arm C (p = 0.002; log rank test). New drugs were used in 60% in arm A, 57% in arm B and 45% in arm C. 4 subjects had a dose modification in the first cycle due to toxicity with 2 interrupting. There were 39 SAEs with 21 in arm C. There were no deaths.
Conclusions: The primary aim of the trial was to gain experience in using IL-2. IL-2 delayed restarting drugs and fewer new drugs were used.
Introduction
Given the toxicities and costs of antiretroviral therapy (ART) [1,2], strategies of CD4 cell count-guided interruption of ART, whereby therapy is interrupted when the CD4 cell count moves above a certain threshold and restarts when a lower threshold is crossed, were under consideration in 1999-2001 [3-5]. At this time, a pilot trial was designed to study ART interruption with the concomitant use of two cycles of interleukin-2 (IL-2) before the interruption (and thereafter as required), aiming to prolong the time before CD4 cell counts fell to levels requiring the reintroduction of ART. This was compared with interrupting without IL-2 and with continuous therapy in an open, randomized, three-arm trial. Results of the SMART trial of CD4 cell count-guided interruption have now shown that there is a clearly increased risk of clinical events with this strategy and hence such interruption strategies are now strongly discouraged [3,6]. Even in this new situation, we consider it relevant to present the results of the pilot trial since it seems inevitable that patients will at times interrupt ART and it is of interest to understand the effects of IL-2 in this context.
The primary aims of this study were (a) to gain experience in ART interruptions in patients with viral suppression and adequate CD4 cell counts, with and without IL-2; (b) to obtain preliminary data on the ability of IL-2 to increase the duration of ART interruption and to maintain the CD4 cell count at > 200 cells/μl; and (c) to evaluate the safety of treatment interruption and the ability of ART, when restarted, to 're-suppress' HIV plasma RNA (viral load).
Methods
Subjects and treatments
Entry criteria for the study were HIV seropositive, over 18 years of age, CD4 cell count ≥ 300 cells/μl and viral load < 200 copies/ml for ≥ 12 weeks on current ART, CD4 cell count nadir > 100 cells/μl, and able to give informed consent.
Participants were randomized in a 1:1:1 ratio to the three arms of the study. Arm A was continuous ART; any changes for whatever reason were done without any interruption in therapy. Arm B was continuation of ART for 9 weeks and then interruption of therapy. The same regimen was restarted if the CD4 cell count fell twice to < 200 cells/μl. Further interruptions were considered if viral load was < 50 copies/ml for ≥ 12 weeks and if the patient's CD4 cell count was greater than that at baseline. Arm C also continued ART for 9 weeks but two cycles of IL-2 were added, one at week 0 and one at week 8. At week 9, ART was interrupted and a new cycle of IL-2 alone given if the CD4 cell count fell to < 300 cells/μl. ART was restarted if the CD4 cell count fell to < 200 cells/μl. Further ART interruptions after two cycles of IL-2 were considered if the patient's viral load was < 50 copies/ml for > 12 weeks and if CD4 cell count was greater than at baseline.
The randomization was stratified by clinical centre and current CD4 cell count (above or below 500 cells/μl), since CD4 cell count was anticipated to influence the length of time before needing to restart ART.
Recombinant IL-2 (Proleukin, Chiron Corp., Emeryville, California, USA) was given as subcutaneous injections of 4.5 X 106 IU (4.5 MIU) every 12 h for 5 days for two cycles at week 0 and week 8. 'Comfort drugs' were provided for the symptomatic treatment of known side effects.
Treatment interruption and follow-up
If subjects were taking a regimen containing a nonnucleoside reverse transcriptase inhibitor, the drug was discontinued 3 days prior to all other antiretroviral drugs; otherwise all drugs were discontinued simultaneously.
Participants attended for follow-up at weeks 4, 9, 11, 13, 17, 21 and 33, and every 12 weeks thereafter until study close (week 105). The study was approved by the Royal Free Hospital Research Ethics Committee as a multicentre pilot study.
Safety and dose modification of interleukin-2
Adverse events were graded according to the modified US Division of AIDS/CPCRA Toxicity table (http://rcc.tech-res.com ). The decision to modify the dosage was based on the clinical judgement of the principal investigator, in discussion, as appropriate, with other investigators. The dose of IL-2 that could be delivered under this protocol from 1.5 to 4.5 MIU.
Results
Between March 2001 and July 2004, 86 patients were recruited: 27 into arm A (continued ART), 28 into arm B (interrupted therapy) and 31 into arm C (interrupted with IL-2). Subjects were mostly white homosexual men with a mean age of 40 years (SD, 7.8). The median baseline CD4 cell count was 754 cells/μl [interquartile range (IQR), 240-1400] and median nadir CD4 cell count was 268 cells/μl (IQR, 62-822). While all had baseline viral load < 200 copies/ml, 88% had baseline viral load < 50 copies/ml; 9% had previous AIDS. Patients had been taking a median of six antiretroviral drugs for a median of 5 years. Four patients recruited were found to be ineligible, two because of baseline CD4 cell counts < 300 cells/μl and two because of a CD4 cell count nadir < 100 cells/μl. Median follow-up was 27 months (IQR, 22-58). No patients were lost to follow-up.
Adherence to strategy TOP
Of the 27 patients in arm A, nine interrupted therapy for a median of 22 weeks (IQR, 2-74) by patient wish and two remained off ART during the study period. Of the 28 patients in arm B, 27 interrupted therapy (one withdrew consent). The interruption occurred at a median of week 9 (IQR, 8-14) as per protocol and the median CD4 cell count at interruption was 694 cells/μl (IQR, 199-1247). The initial interruption lasted a median of 50 weeks (IQR, 3-144) and 20 subjects restarted ART with a median CD4 cell count of 293 cells/μl (IQR, 130-917). Five subjects had a second and one a third ART interruption. One patient restarted ART owing to severe thrombocytopenia.
In arm C, 31 patients interrupted therapy at week 9 (IQR, 8-25). All 31 subjects had at least one cycle of IL-2 (29 had two, 9 had three, 4 had four and 3 had five cycles). The median starting dose was 4.5 MIU; six subjects had a dose modification and three patients interrupted an IL-2 cycle. The median CD4 cell count at ART interruption was 1810 cells/μl (IQR, 683-4817), and ART interruption lasted a median of 96 weeks (IQR, 11-169). Eighteen patients restarted HAART, with a median CD4 cell count of 296 cells/μl (IQR, 34-469), and four subjects had a CD4 cell count of < 200 cells/μl. Two patients had a second and one had a third ART interruption.
Time to reinitiation of antiretroviral therapy
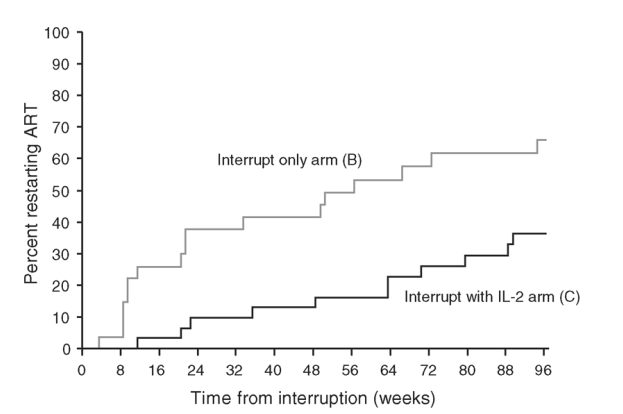
Figure 1 shows Kaplan-Meier estimates of the proportion of people in arm B and arm C who restarted ART according to time since interrupting ART. By week 105, there was a 66% probability of having restarted ART in arm B compared with 34% in arm C (log rank P = 0.002).
Fig. 1. Kaplan-Meier estimates of cumulative percentage restarting antiretroviral therapy by time from interruption.
Changes in viral load and CD4 cell count during follow-up
While baseline CD4 cell counts were similar for patients in each of the three study arms (Fig. 2), there was a significant difference (Kruskall-Wallis analysis) in median CD4 cell count at week 9 (-10, -30 and +321 cells/μl in arms A, B and C, respectively; P < 0.0001) and week 105 (-24, -212 and -268 cells/μl in arms A, B and C, respectively; P = 0.0002). The much higher CD4 cell count in arm C at week 9 reflects the marked effect of IL-2. At week 105, the difference in CD4 cell count change between arms was largely driven by greater declines in both the interruption arms (median 212 and 268 cells/μl in arms B and C, respectively), compared with little change in the continuation arm (median decline of 24 cells/μl). One CD4 cell count measurement < 150 cells/μl occurred in two, four and three patients, respectively, in arms A, B and C. Only two patients, both in arm C, had a confirmed second consecutive value < 150 cells/μl. In addition, three patients in arm C, but none in the other arms, experienced a CD4 cell count < 100 cells/μl.
Median viral load values were higher from week 9 in the two interruption arms. Arm C had higher viral load levels than B, presumably related to the greater proportion not taking ART at the time. At week 105, there was a highly significant difference between arms: 1.7 log copies/ml, (IQR, 1.7-2.3), 2.3 log copies/ml (IQR, 1.7-4.4) and 4.2 log copies/ml (IQR, 2.9-4.6) in arms A, B and C, respectively (Kruskall-Wallis P < 0.0001), with the interruption arms having much higher levels than the continuation arm.
Therapy was restarted after interruption in 20 subjects in arm B and 18 in arm C. Of these, 19 and 16, respectively, had follow-up at 24 weeks; 94% in arm B and 91% in arm C had viral load < 200 copies/ml.
Use of new antiretroviral drugs
New drugs were used in 54% of patients overall: 60% in arm A, 57% in arm B and 45% in arm C (P = 0.12). Another class of drug was added to the regimen in 11% of patients in arm A compared with 4% in arm B and 3% in arm C (P = 0.15).
Adverse and clinical events
There were 39 grade 3 and 4 adverse events, with 21 in arm C: hepatotoxicity (12 patients), gastrointestinal toxicity (6), haematological toxicity (6), systemic effects (5), biochemical dysfunctions (3), respiratory toxicity (2), neurological toxicity (2), and cardiovascular, skin and renal toxicity (1 each). There were no AIDS-defining events and no deaths in the study.
Discussion
The SMART study had a profound impact on ART interruption [7]. Part of the reason for the raised hazard in the treatment interruption arm was related to the reduction in CD4 cell count during the treatment interruption [3]; immunotherapy with IL-2 could raise the CD4 cell count and so potentially reduce these risks [8-10]. This pilot study looked at the feasibility of using IL-2 to prolong an ART interruption. Compared with patients who interrupting treatment without IL-2, there was a significantly longer time to restarting ART in those who received IL-2. A starting dose of IL-2 4.5 MIU twice daily for 5 days with concomitant ART was well tolerated with little need for dose reduction. It is, however, disturbing that in some cases the interruption with IL-2 was allowed to continue such that the CD4 cell count fell to < 200 cells/μl. While this may be a risk with any interruption, it is difficult to rule out that it specifically related to interruption with IL-2. Participant bias may be a factor owing to the open nature of the study. It may be that those who interrupted ART with IL-2 were kept off ART for longer periods than those who interrupted without IL-2 despite the slightly poorer CD4 cell count outcomes.
SMART and other trials suggest that treatment interruption should be discouraged strongly [6,7]. However, patients continue to express the wish to interrupt therapy against such advice [11] and, while the clinical benefit associated with CD4 cell count rises with IL-2 awaits evaluation [12], use of IL-2 may be considered in some cases to help to maintain a higher CD4 cell count.
|
|
| |
| |
|
|
|