| |
Major Clinical Outcomes in Antiretroviral Therapy (ART)-Naive Participants and in Those Not Receiving ART at Baseline in the SMART Study
|
| |
| |
The Strategies for Management of Antiretroviral Therapy (SMART) Study Group
The Journal of Infectious Diseases April 15, 2008;197:1133-1144
ABSTRACT
Background. The SMART study randomized 5472 human immunodeficiency virus (HIV)-infected patients with CD4+ cell counts >350 cells/μL to intermittent antiretroviral therapy (ART; the drug conservation [DC] group) versus continuous ART (the viral supression [VS] group). In the DC group, participants started ART when the CD4+ cell count was <250 cells/μL. Clinical outcomes in participants not receiving ART at entry inform the early use of ART.
Methods. Patients who were either ART naive (n=249) or who had not been receiving ART for >6 months (n=228) were analyzed. The following clinical outcomes were assessed: (i) opportunistic disease (OD) or death from any cause (OD/death); (ii) OD (fatal or nonfatal); (iii) serious non-AIDS events (cardiovascular, renal, and hepatic disease plus non-AIDS-defining cancers) and non-OD deaths; and (iv) the composite of outcomes (ii) and (iii).
Results.
A total of 477 participants (228 in the DC group and 249 in the VS group) were followed (mean, 18 months).
For outcome (iv), 21 and 6 events occurred in the DC (7 in ART-naive participants and 14 in those who had not received ART for 6 months) and VS (2 in ART-naive participants and 4 in those who had not received ART for 6 months) groups, respectively.
Hazard ratios for DC vs. VS by outcome category were as follows: outcome (i), 3.47 (95% confidence interval [CI], 1.26-9.56; p=.02); outcome (ii), 3.26 (95% CI, 1.04-10.25; p=.04); outcome (iii), 7.02 (95% CI, 1.57-31.38; p=.01); and outcome (iv), 4.19 (95% CI, 1.69-10.39; p=.002).
Conclusions. Initiation of ART at CD4+ cell counts >350 cells/μL compared with <250 cells/μL may reduce both OD and serious non-AIDS events. These findings require validation in a large, randomized clinical trial.
BACKGROUND
The most recent randomized clinical trial that addressed the question of when to start antiretroviral therapy (ART) for the treatment of HIV disease was the Concorde study [1]. Since then, despite enormous advances afforded through the development of antiretroviral drugs and the use of combination regimens, there has been no randomized study to address this critically important issue. The absence of randomized data in both the developed and developing world has required antiretroviral treatment guidelines to rely on the interpretation of nonrandomized studies and expert opinions [2, 3]. Current recommendations for the initiation of ART are conservative because of the uncertainty about the risk-benefit ratio associated with earlier ART use at higher CD4+ cell counts. These include toxicities, cost-effectiveness, quality of life issues, adherence, and drug resistance. At present, the initiation of ART is recommended for asymptomatic patients with CD4+ cell counts between 201 and 350 cells/μL or, in developing/resource-poor countries, when the CD4+ cell count has declined to <200 cells/μL.
Data from cohort studies indicate that the risk of AIDS decreases with increasing CD4+ cell counts but nevertheless persists at CD4+ cell counts >500 cells/μL [4]. Furthermore, at any given CD4+ cell count, the risk of AIDS appears to be lower in patients treated with ART than in those who are not [5], and the risk of AIDS or death declines with the start of therapy, even among those with high CD4+ cell counts at initiation, compared with that in patients not receiving ART [6, 7]. Importantly, cohort studies also indicate that risks of serious non-AIDS-related diseases, including cardiovascular, renal, and hepatic conditions and non-AIDS malignancies, are less common at higher CD4+ cell counts [8-13]. Data from the SMART study are consistent with these observations insofar as the rates of AIDS and serious non-AIDS events were significantly higher in participants randomized to the intermittent ART group than in those randomized to the continuous ART group [14].
Here, we report the major clinical outcomes for a subgroup of participants enrolled in SMART who were either ART naive or who had not been receiving ART for >6 months before randomization. This subgroup provides a randomized comparison between early ART at a CD4+ cell count of >350 cells/μL versus deferred ART until the CD4+ count declines to <250 cells/μL. Subgroup analyses for major clinical outcome according to ART history were proposed a priori. However, the analyses presented here are post hoc. Thus, we regard our results as hypothesis generating.
Discussion
The randomization in the SMART study for this patient subset directly compared the early use of ART (at >350 CD4+ cells/μL) versus the deferred use of ART (at <250 CD4+ cells/μL). To our knowledge, the data presented here represent the first substantive evidence from a randomized trial to inform this critical issue in HIV medicine since 1994 [1]. During follow-up, there was substantially lower exposure to ART in the deferred treatment group (DC). The deferred use of ART was consistent with the protocol in the majority of participants; although the DC strategy included discontinuation of ART when the CD4+ cell count increased to >350 cells/μL, 92% of accrued follow-up was in participants who were either not receiving ART or taking ART for the first time after randomization. In the early ART group (VS), most participants (re)initiated ART with CD4+ cell counts between 350 and 549 cells/μL. We combined data from participants who were ART naive and those who had not been receiving ART for at least 6 months before randomization. Had we been more conservative and included only participants who had not been receiving ART for >12 months before randomization, we would have reduced the overall number of composite events from 27 (21 in DC and 6 in VS participants) to 23 (17 in DC and 6 in VS participants). The calculated HR(DC/VS) would still have indicated a significant benefit for the VS strategy (HR, 3.1 [95% CI, 1.2- 7.9]).
The results suggest that morbidity and mortality associated with HIV is probably higher than previously thought for patients with earlier stages of HIV disease. In DC participants, the rates of OD and serious non-AIDS events were similar. The risk of a composite outcome was >4-fold higher in DC participants than in VS participants. The data indicate that the risk of such events is reduced by early versus deferred use of ART (the absolute risk of an OD or serious non-AIDS event in DC versus VS participants was 7.0 vs. 1.6/100 person-years). This difference in absolute risk, if confirmed in a larger trial, is important. Treatment guidelines for the initiation of ART are conservative because the risk of AIDS (OD in our categories) at high CD4+ cell counts is relatively low and because ART-related toxicities (and other factors) have been assumed to outweigh any likely ART-related benefits. Our data suggest that early ART use may result in substantial reductions in serious non-AIDS events. Relative to the DC group, 289 additional person-years of ART prevented 20 serious clinical events in the VS group. Hence, prevention of 1 event requires 14 extra person-years of ART. When assessed for AIDS events alone, prevention of 1 event requires 42 extra person-years of ART.
The occurrence of AIDS at higher than expected CD4+ cell counts has been described previously [7, 17]. There are fewer data linking CD4+ cell counts and the risk of non-AIDS-related morbidity and mortality. Some non-AIDS conditions appear to be linked to progressive immunodeficiency [8, 9, 18]. In the SMART study, lower CD4+ cell counts and higher plasma HIV-RNA levels explain much of the excess risk in DC compared with VS participants for non-OD death as well as OD [18]. The data presented here for SMART participants not receiving ART are consistent with these observations.
Mechanistically, it appears to be unlikely that a common pathway for all non-AIDS events exists. Furthermore, the risk and/or the benefit of ART for reducing non-AIDS diseases is not clear. For example, PI therapy has been associated with an increased risk of myocardial infarction [19, 20]. On the other hand, continuous ART in SMART was associated with better cardiovascular outcomes than ART interruption [14]. It is possible that untreated HIV disease supports a range of inflammatory responses that, particularly over long periods, set the scene for atherosclerosis in individuals with additional risk factors for poor cardiovascular outcomes. The increased risk of cardiovascular disease in patients with systemic lupus erythematosus is similar in this regard [21, 22]. In addition, in SMART, changes in lipids appear to explain some of the increased risk of cardiovascular disease in the DC compared with the VS group [23]. The pathogenesis of hepatic and renal disease along with non-AIDS cancer is less clear. These equivocal and perhaps controversial issues further highlight the need for a careful evaluation of risks and benefits of early treatment with ART [24]. Furthermore, they reinforce the importance of studies with systematically collected clinical end points matched with appropriate biological samples as a key feature of trial design for HIV infection [25].
There are limitations to our findings. First, we did not prespecify the patient subgroup or composite outcome of OD and serious non-AIDS events in the original SMART analysis plan. Thus, our findings generate new hypotheses that require confirmation. Predefined components of our composite outcome have been reported for all SMART participants [14, 26]. For each end point, there is an excess risk in the DC compared with the VS group. The higher risk in DC compared with VS participants for this composite outcome is not limited to those in this small subgroup. The HR(DC/VS) for the composite of OD and serious non-AIDS events is >1 for each of the 4 cohorts classified by baseline ART use and is and significantly >1 for all SMART participants. Second, although the CIs around the HR estimates are wide as a result of the small number of composite events (n=27), they are qualitatively similar to the reliably estimated 2-fold difference in risk for the overall SMART study. Third, we included a small number of participants who had prior AIDS-defining illnesses (23 DC and 29 VS participants). However, analyses that excluded these participants were consistent with the present results (table 3). Finally, in SMART we did not formally collect information on treatment-limiting toxicities that did not attain the severity to qualify as a grade 4 adverse events. During the period of observation, there were 3 DC participants (1%) and 24 VS participants (10%) who ceased ART with toxicities being the reason cited. During follow-up, there were 25 and 18 grade 4 events in the DC and VS groups, respectively.
These data indicate that the absolute risk of serious non-AIDS events in untreated patients is greater than the risk of AIDS at high CD4+ cell counts. They reveal that risk reductions for AIDS associated with the earlier use of ART appear to be greater than previously estimated. Furthermore, the data suggest that reduced risk for serious non-AIDS outcomes arising from the early use of ART might substantially outweigh any increased risk of serious non-AIDS outcomes associated with the use of ART. We believe the data presented here provide a strong clinical rationale for the design and conduct of a randomized trial to estimate precisely the risks and benefits of the initiation of ART before the CD4+ cell count declines to <350 cells/μL for the treatment of chronic HIV disease.
Methods
Participants.
Participants were included if they were randomized in the SMART study and were either ART naive or satisfied the following eligibility criteria for having been previously treated but not receiving ART for a minimum of 6 months before randomization (hereafter, "not receiving ART"): (1) were not receiving ART at the time of randomization in SMART; (2) had at least 1 plasma HIV RNA assessment during the 6 months before randomization; and (3) all plasma HIV RNA levels in the 6 months before randomization were >10,000 copies/mL. We excluded patients who had not been receiving ART for <6 months before randomization to avoid the possible short-term effects of ceasing ART (e.g., the steeper decline in CD4+ cell count). The principal eligibility criterion for the SMART study was a CD4+ cell count of >350 cells/μL. Other eligibility criteria for SMART are described elsewhere [14].
Randomization.
Participants were randomly allocated in equal proportions to 1 of 2 treatment strategies: continuous ART (the viral supression [VS] group) and intermittent ART (the drug conservation [DC] group). For the present analyses, this randomization corresponded to the immediate (re)initiation of ART after randomization at CD4+ cell counts >350 cells/μL (the VS group) versus the deferred (re)initiation of ART when either the CD4+ cell count declined to <250 cells/μL, the CD4+ cell percentage declined to <15%, or symptoms of HIV disease developed (the DC group).
Assessments.
Before randomization, participantsf ART and medical history were obtained along with nadir and baseline CD4+ cell counts and plasma HIV RNA levels. Routine visits occurred at 1 and 2 months, every 2 months thereafter for the first year, and every 4 months in subsequent years. Visits included clinical assessments, and samples were obtained for measurement of CD4+ cell count and plasma HIV RNA level. At baseline and annually, a 12-lead electrocardiogram was obtained, and data were electronically transmitted to a central reading facility for assessment of silent myocardial infarction [15].
End-point definitions.
Definitions for the primary end point of opportunistic disease (OD) or death from any cause (OD/death) and the major secondary composite end point of cardiovascular-, renal-, and hepatic-disease events are given in the online-only appendix of the primary-results article [14]. In the present report, 4 major clinical outcomes are considered: (i) the SMART primary end point (OD/death); (ii) OD (fatal or nonfatal); (iii) serious non-AIDS events (cardiovascular, renal, and hepatic disease plus non-AIDS-defining cancers [excluding nonmelanoma skin cancers] and deaths from non-OD causes); and (iv) the composite of outcomes (ii) and (iii). In the end-point categories (ii) and (iii), deaths due to OD and all causes except OD are counted. Thus, the composite of (ii) and (iii) can be viewed as major morbidity (OD and non-OD) or all-cause mortality. As previously reported, an end-point review committee, blinded to treatment group, reviewed all major nonfatal clinical events [14]. The end-point review committee also classified the underlying cause of death using the system of the Coding of Death in HIV Project [16]. Grade 4 adverse events were defined as potentially life-threatening symptomatic events according to the Division of AIDS common toxicity table.
Statistical analysis.
All analyses were intention to treat. Data through 11 January 2006 were reported. On that date, following the recommendations of an independent data and safety monitoring board, all participants in the DC group were advised to (re)initiate ART. Thus, time-to-event analyses are censored at the earliest of the date of death, the lost to follow-up date, or 11 January 2006.
The time from randomization to first event was compared between the DC and VS groups by use of Kaplan-Meier plots for each event category. For patients reporting multiple events in a single outcome category, only the first event was included. For patients reporting multiple events in different outcome categories, each first event in each category was included. The hazard ratio for DC vs. VS-hereafter, HR(DC/VS)-was estimated by fitting a Cox model with a single binary indicator for treatment group overall and separately in ART-naive and ART-experienced participants. Differences between these 2 groups were assessed by including an interaction term in the Cox model. Randomization protects these comparisons between treatment groups from confounding.
Person-years at specific CD4+ cell counts were counted according to the most recently measured or time-updated value (latest CD4+ cell count), and rates per 100 person-years for the composite outcome were determined for DC and VS participants. Randomization does not protect these comparisons between treatment groups from confounding.
Throughout, P values refer to 2-sided tests. Because the subgroup and the serious non-AIDS outcomes considered in this report were not prespecified, we recommend cautious interpretation of P values.
Results
Patient population.
A total of 477 participants (8.7%) in the SMART study were either ART naive (n=249) or had not been receiving ART for >6 months before randomization (n=228) and are included in these analyses. The relationship of these participants to the total SMART cohort is summarized in figure 1.
Figure 1. Selection of the analyzed cohort (antiretroviral therapy [ART] naive or not receiving ART for >6 months) from the overall population of SMART participants. DC, drug conservation; VS, viral supression.
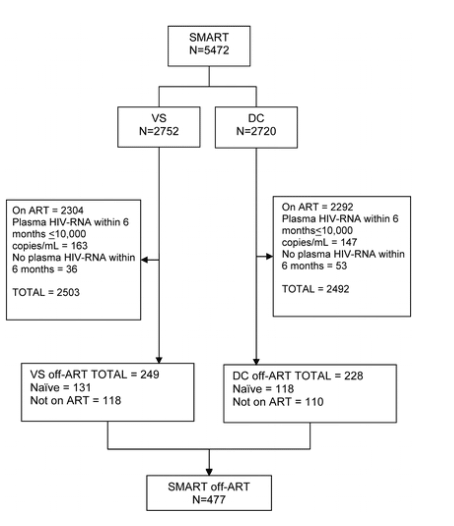
Table 1 shows the baseline characteristics of the 228 and 249 participants who were respectively randomized into the DC and VS groups. Treatment groups were well balanced for all baseline characteristics. One-hundred eighteen participants (52%) and 131 participants (53%) were ART naive in the DC and VS groups, respectively. The median baseline CD4+ cell count and plasma HIV RNA level for the cohort were 447 cells/μL and 4.5 log10 copies/mL. The median nadir CD4+ cell count was 361 cells/μL.
Table 1. Overall baseline characteristics and those for patients who were antiretroviral therapy (ART) naive or who had not received ART for 6 months (no ART) before randomization in the SMART study.
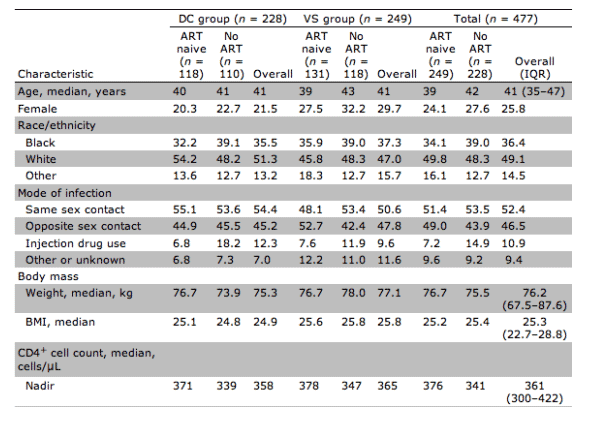
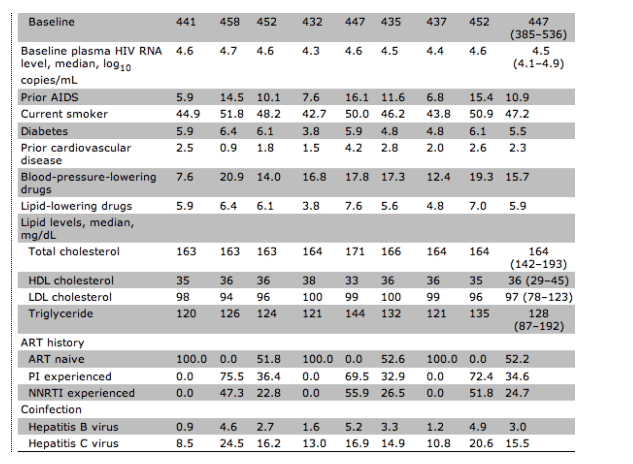
The median duration of ART exposure in previously treated participants was 4 years. Approximately 75% had used an HIV protease inhibitor (PI), for a median duration of 2.5 years. Approximately 50% had used a nonnucleoside reverse-transcriptase inhibitor (NNRTI), for a median duration of 2.5 years. All previously treated participants had used a nucleoside reverse-transcriptase inhibitor (NRTI).
In the VS group, (re)initiation of ART comprising NRTIs in combination with NNRTIs, NRTIs in combination with a PI, and NRTIs alone was reported for 134 (56%), 73 (31%), and 31 (13%) participants, respectively.
There were modest differences in baseline characteristics between participants who were ART naive and those who were experienced. ART-naive participants were on average 3 years younger, less likely to be current smokers, less likely to be taking blood-pressure-lowering or lipid-lowering therapy, and had slightly lower serum triglyceride levels. They were also less likely to be coinfected with either hepatitis B or C virus and less likely to have had a prior AIDS-defining illness.
The mean period of follow-up was 18 months (median, 15 months). Eleven (4 DC and 7 VS) participants were lost to follow-up.
Exposure to ART.
DC and VS participants received ART for 18% and 89% of the follow-up time, respectively. In the DC group, 68 participants (30%) (re)initiated ART, 25 of whom were ART naive (21% of the ART-naive participants) and 43 of whom were ART experienced (39% of the ART-experienced participants). Reasons for (re)initiation of ART included CD4+ cell count decline to <250 cells/μL (38 participants [56% of those who (re)initiated ART]); CD4+ cell percentage decline to <15% (28 participants [41%]); symptoms of HIV disease or development of OD (14 participants [21%]), with 4 participants developing an OD event; and nonprotocol reasons (14 participants [21%]). Of these 68 participants, 30 (44%) initiated ART at a CD4+ cell count 250 cells/μL. Kaplan-Meier estimates for DC participants (re)initiating ART were 11%, 25%, 39%, 45%, 47%, and 55% at 6, 12, 18, 24, 30, and 36 months, respectively. In the DC group, participants (re)initiated ART at a median CD4+ cell count of 236 cells/μL (interquartile range [IQR], 183-310 cells/μL); those who were ART naive initiated ART at median of 245 cells/μL (IQR, 192-338 cells/μL), versus 225 cells/μL (IQR, 180-307 cells/μL) among ART-experienced participants. In comparison, 238 participants (96%) in the VS group (re)initiated ART at study entry, at a CD4+ cell count 200 cells/μL higher; 189 (79%) had a CD4+ cell count between 350 and 549 cells/μL, and 49 (21%) had a CD4+ cell count >550 cells/μL.
In the DC group, 68 (30%) of 228 participants started ART, and 27 (12%) subsequently interrupted ART. The majority of follow-up time was accrued before the first (re)initiation of ART: 236 person-years (79%) accrued from study entry to first (re)initiation of ART, 39 person-years (13%) from (re)initiation of ART to discontinuation, and 25 person-years (8%) after discontinuation of ART. During this 8% of follow-up time, 3 participants in the DC group experienced an OD event, a serious non-AIDS event, or died.
CD4+ cell count and plasma HIV RNA outcomes.
Reflecting the differing ART exposures, there were substantial differences in CD4+ cell counts and plasma HIV RNA levels over time between the DC and VS groups. Figure 2 shows time curves for CD4+ cell count and plasma HIV RNA level for each treatment group. The CD4+ cell count was, on average, 148 cells/μL lower in DC than VS participants. The proportions of participants with plasma HIV RNA levels 400 copies/mL at 12 and 24 months were 10% and 25% in the DC group and 59% and 56% in the VS group.
Clinical outcomes.
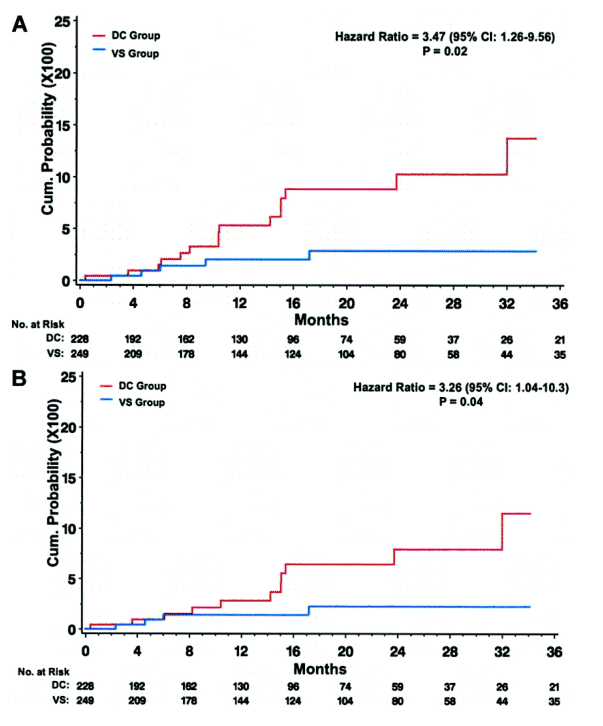
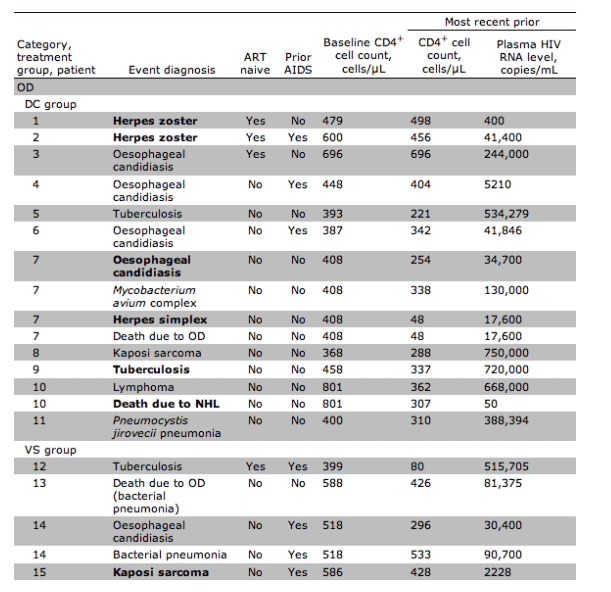
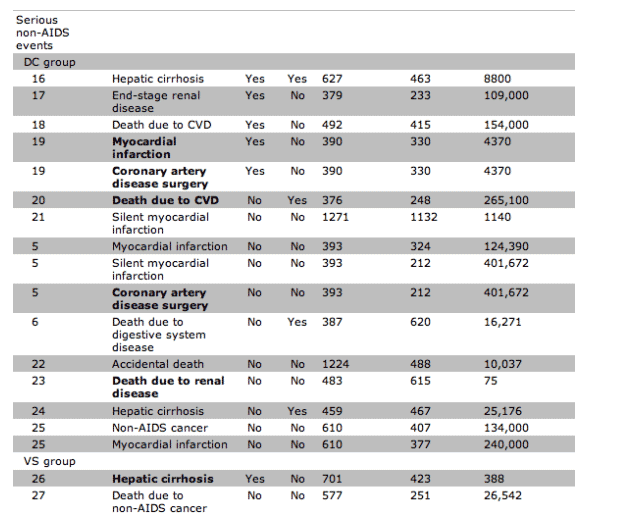
Fifteen participants in the DC group and 5 in the VS group experienced OD/death (HR, 3.5 [95% confidence interval {CI}, 1.3-9.6) (figures 3 and 4A). The corresponding HR(DC/VS) for OD and for serious non-AIDS events was 3.3 (95% CI, 1.0-10.3) and 7.0 (95% CI, 1.6-31.4), respectively (figures 3, 4B, and 4C). There was relatively little overlap in OD and serious non-AIDS events (table 2); 21 participants in the DC group and 6 in the VS group experienced at least 1 of these (the composite end point) (figures 3 and 4D). The HR for the composite outcome was 4.2 (95% CI, 1.7-10.4). The excess risk (DC minus VS) was 5.4 events/100 person-years (95% CI, 3.4-7.4). The cumulative percentage developing the composite end point by 12, 24, and 36 months were 8%, 13%, and 18% for DC participants and 2%, 4%, and 4% for VS participants (figure 4D).
Figure 3. Hazard ratios (HRs) for clinical outcomes among the overall cohort and among those who were either antiretroviral therapy (ART) naive or not receiving ART at baseline. Horizontal lines represent the 95% confidence interval for each HR. The P value for interaction compares event rates within each category for ART-naive and ART-experienced participants. DC, drug conservation; VS, viral supression.
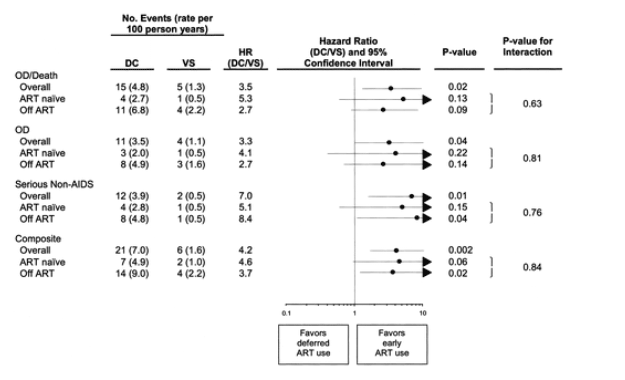
Figure 4. Kaplan-Meier time curves for cumulative probability of opportunistic disease (OD) and death (A), OD alone (B), serious non-AIDS events and non-OD death (C), and the composite of OD and serious non-AIDS events, which includes all-cause death (D). The solid red lines indicate events in participants in the drug conservation (DC) group, and the dashed blue lines indicate events in participants in the viral supression (VS) group.
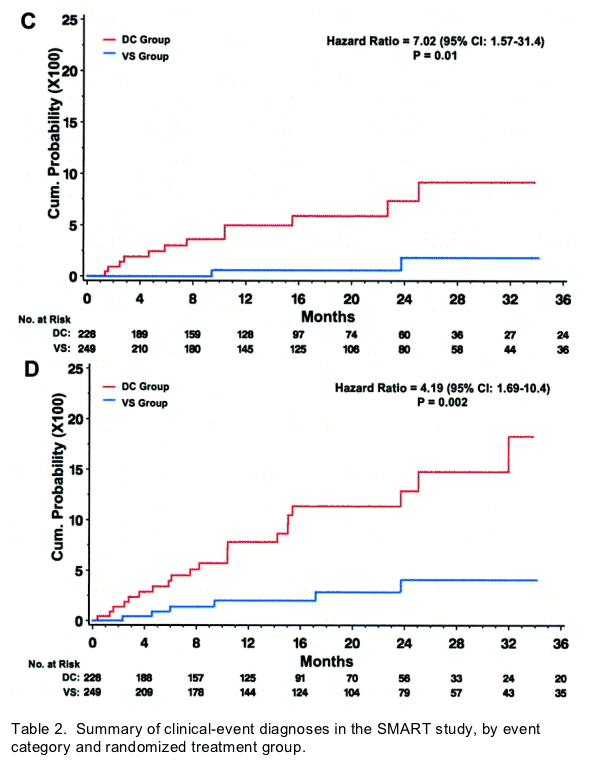
For the composite end point, HRs were 4.6 (95% CI, 1.0-22.2) for the ART-naive participants and 3.7 (95% CI, 1.2-11.2) for the ART-experienced participants (p=.84 for difference in these HRs). In participants excluded from this cohort (see figure 1), HRs for the composite end point were 1.3 (95% CI, 0.6-3.2) for experienced participants not receiving ART at entry (200 DC and 199 VS participants) and 2.0 (95% CI, 1.5-2.6) for participants receiving ART at entry (2292 DC and 2304 VS participants) (p=.27 for the difference in HRs between these 4 subgroups). In the entire SMART study, there were 270 participants with a composite end point (180 DC and 90 VS participants), with a corresponding HR of 2.1 (95% CI, 1.6-2.7).
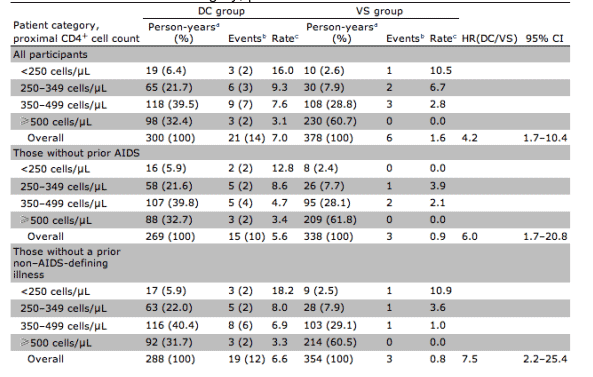
Table 3 summarizes the composite outcome by treatment group and proximal CD4+ cell count. Event rates were higher during the time when participants experienced lower CD4+ cell counts. Per design, participants in the DC group, relative to those in the VS group, spent substantially longer periods of time with CD4+ cell counts in the <350 cells/μL stratum (28% vs. 11% of the time) and substantially less time with CD4+ cell counts in the >500 cells/μL stratum (32% vs. 61% of the time). The majority of events in the DC group (57%) occurred at proximal CD4+ cell counts >350 cells/μL. Nine (75%) of 12 events in the DC group (1 OD and 8 serious non-AIDS events) that occurred at CD4+ cell counts <350 cells/μL happened before the (re)initiation of ART. Table 3 also summarizes the composite outcome in the subgroup of participants without prior AIDS and without a prior non-AIDS-defining illness, for which the HR(DC/VS) was 6.0 (95% CI, 1.7-20.8) and 7.5 (95% CI, 2.2-25.4), respectively. Although event rates were similar or reduced compared with the overall cohort, the HR(DC/VS) did not decrease after excluding participants with these prior events.
Table 3. Clinical events (composite end point), by treatment group and proximal CD4+ cell count. Patient category, proximal CD4+ cell count
We investigated whether differences in nadir CD4+ cell counts might have affected the distribution of clinical events. The data in table 1 indicate that median nadir CD4+ cell counts were not substantially different for the ART-naive or previously treated participants between treatment groups. The differences between ART-experienced and ART-naive participants with respect to median baseline CD4+ cell count (437 and 376 cells/μL, respectively) and median nadir CD4+ cell count (452 and 341 cells/μL, respectively) participants were not appreciable. The HR(DC/VS) for our primary outcome was unaffected in analyses that did or did not adjust for nadir CD4+ cell count (HR of 3.7 in both analyses).
Grade 4 events were experienced by 25 DC participants and 18 VS participants. The HR(DC/VS) was 1.6 (95% CI, 0.9-3.0).
|
|
| |
| |
|
|
|