| |
Diabetes drugs (glitazones) double fracture risk: Swiss study
|
| |
| |
008-04-29
LONDON (Reuters) - Diabetes drugs Avandia from GlaxoSmithKline Plc and Takeda Pharmaceutical Co Ltd's Actos can more than double the risk of bone fractures, Swiss researchers said on Monday.
Previous studies have established a heightened risk for fractures among patients taking both medicines, since they may cause slower bone formation and faster bone loss.
The new research sheds light on the scale of the problem by comparing the records of 1,020 diabetic patients with fractures diagnosed by British doctors between 1994 and 2005 against a control group of diabetics who did not have fractures.
Christian Meier of University Hospital Basel found those on Avandia or Actos -- known generically as rosiglitazone and pioglitazone -- had double or triple the odds of non-spine fractures.
The odds for fracture were increased among patients who took the drugs for approximately 12 to 18 months and the risk was highest for those with two or more years of therapy.
Fractures of the hip and wrist were most notable, according to the findings published in the journal Archives of Internal Medicine.
Avandia and Actos belong to a class of medicines called thiazolidinediones, which are designed to sensitise the body to insulin. But researchers believe they also stimulate the action of a cell that drives the process of reabsorbing bone, making bones more apt to break.
Both are blockbuster products but Avandia has lost out to Actos following publication of a U.S. study last May linking it to heart attack risk.
Use of Thiazolidinediones and Fracture Risk
Arch Intern Med. April 28, 2008;168(8):820-825.
Christian Meier, MD; Marius E. Kraenzlin, MD; Michael Bodmer, MD; Susan S. Jick, DSc; Hershel Jick, MD; Christoph R. Meier, PhD
Author Affiliations: Division of Endocrinology, Diabetes, and Clinical Nutrition (Drs C. Meier and Kraenzlin) and Basel Pharmacoepidemiology Unit, Division of Clinical Pharmacology and Toxicology (Drs Bodmer and C. R. Meier), University Hospital Basel, Basel, Switzerland; and Boston Collaborative Drug Surveillance Program, Boston University School of Medicine, Lexington, Massachusetts, and Department of Epidemiology, School of Public Health, Boston University, Boston, Massachusetts (Drs S. S. Jick, H. Jick, and C. R. Meier).
"....In summary, this nested case-control analysis of diabetic patients provides additional evidence that the use of thiazolidinediones for approximately 12 or more months may increase the risk of osteoporotic nonvertebral fractures..... The findings of this large, population-based, nested, case-control analysis provide further evidence that current use of rosiglitazone and pioglitazone in women and men with type 2 DM may be associated with an approximately 2- to 3-fold increased risk of hip and nonvertebral osteoporotic fractures...."
ABSTRACT
Background: Thiazolidinediones may adversely affect the skeleton owing to decreased bone formation and accelerated bone loss.
Methods: This study examines the association between the use of thiazolidinediones or other oral antidiabetic drugs and the risk of fracture. This nested case-control analysis uses the UK General Practice Research Database, including case patients with fracture aged 30 to 89 years with an incident fracture diagnosis between January 1994 and December 2005 and control subjects who were matched to case patients on age, sex, calendar time, and general practice attended. We assessed the odds ratios (ORs) of having a fracture associated with the use of rosiglitazone maleate, pioglitazone hydrochloride, other oral antidiabetic agents, or insulin.
Results: There were 1020 case patients with an incident low-trauma fracture and 3728 matched controls. After adjustment for age, body mass index, other antidiabetic drugs, comedication, and comorbidities, the ORs for users of 8 or more thiazolidinedione prescriptions (corresponding to approximately 12-18 months of therapy) compared with nonuse was 2.43 (95% confidence interval [CI], 1.49-3.95). Rosiglitazone (OR, 2.38; 95% CI, 1.39-4.09) and pioglitazone (OR, 2.59; 95% CI, 0.96-7.01) were used more frequently by case patients with fracture (predominantly hip and wrist fractures) than by controls. The association was independent of patient age and sex and tended to increase with thiazolidinedione dose. No materially altered relative fracture risk was found in association with the use of other oral antidiabetic drugs.
Conclusion: This analysis provides further evidence of a possible association between long-term use of thiazolidinediones and fractures, particularly of the hip and wrist, in patients with diabetes mellitus.
COMMENT
Preclinical14, 16-18 and clinical19-21 studies indicate that thiazolidinedione (glitazone) use may have adverse effects on bone metabolism, resulting in reduced osteoblastic bone formation and accelerated bone loss. The findings of this large, population-based, nested, case-control analysis provide further evidence that current use of rosiglitazone and pioglitazone in women and men with type 2 DM may be associated with an approximately 2- to 3-fold increased risk of hip and nonvertebral osteoporotic fractures. The numbers of vertebral and rib fractures were too low for a meaningful assessment of fracture risk in relation to thiazolidinedione use. Although short-term exposure to thiazolidinediones did not materially alter the relative risk estimate of developing a fracture, the OR was increased for current users of 8 or more prescriptions, corresponding to approximately 12 to 18 months of therapy. The highest risk estimate was seen in users of 15 or more prescriptions, corresponding to 2 or more years of therapy. Fracture risk was independent of BMI, comorbidities, diabetic complications, DM duration, and use of other oral antidiabetic drugs.
Treatment with thiazolidinediones has recently been reported to possibly increase the risk of fractures in a randomized trial22 exploring the efficacy of rosiglitazone, metformin, or glyburide encompassing 4360 patients with type 2 DM. The authors22 of A Diabetes Outcome Progression Trial (ADOPT) reported an unexpected observation that was not part of the prespecified analysis plan: more women taking rosiglitazone experienced fractures of the upper or lower limbs compared with women taking the comparison antidiabetic drugs. Furthermore, findings from clinical trial databases23-24 underline an excess of fractures in patients treated with pioglitazone, suggesting that the increased fracture risk may be a class effect of thiazolidinediones. For pioglitazone, the incidence was 1.9 fractures per 100 patient-years in the pioglitazone-treated group compared with 1.1 fractures in the comparator-treated group, which translates to an excess risk of 0.8 fractures per 100 patient-years of use for women taking the drug.24 The present findings support this recent observation and indicate a possible class effect of thiazolidinediones because current use of 8 or more prescriptions of rosiglitazone (OR, 2.38; 95% CI, 1.39-4.09) or pioglitazone (OR, 2.59; 95% CI, 0.96-7.01) was associated with increased nonvertebral fracture risk.
Recently published data indicate that thiazolidinedione-associated fractures are restricted to the lower limb (foot, ankle, fibula, and tibia) and upper limb (forearm, hand, and wrist)22 despite the fact that in healthy postmenopausal women20 and older diabetic patients19, 21 bone loss during thiazolidinedione use was observed at sites related to osteoporotic fractures (ie, the lumbar spine and the femoral neck). This study was particularly designed to investigate the association between the use of thiazolidinediones and fractures classically related to osteoporosis. In contrast to the findings by Kahn et al,22 we found that thiazolidinedione use may also affect fractures at the femoral neck. Apart from methodological issues, the age of the study population might explain differences in the types of fractures observed: the average age of participants in ADOPT was in the middle 50s, whereas in the present cohort approximately 60% were older than 60 years at the index date. Because the incidence of fractures at the spine and hip increases with age, previous findings in younger individuals with fractures at the lower and upper distal limbs might reflect the kinds of fractures that younger women would tend to experience.
Furthermore, it may be speculated that thiazolidinediones might have differential effects on bone with respect to patient age. Rosiglitazone-induced bone loss in adult and old animals seems to occur via distinct cellular mechanisms. Although bone loss in adult mice was associated with a decreased number of osteoblasts and a decreased bone formation rate, bone loss in older animals was a direct result of increased osteoclast numbers.32 If confirmed in further studies, these findings could turn out to be particularly relevant because increasing numbers of young patients are being considered for treatment with thiazolidinediones. To our knowledge, no data are available on the effect of thiazolidinediones on the acquisition of peak bone mass in overweight and insulin-resistant teenagers receiving thiazolidinediones.
Thiazolidinediones are ligands for peroxisome proliferator-activated receptor {gamma} (PPAR{gamma}), a family of nuclear receptors that regulate gene transcription; PPAR{gamma}s are most abundant in adipocytes and regulate their differentiation and function. When added to bone marrow cultures, it has been demonstrated that rosiglitazone stimulates adipogenesis and inhibits osteoblastogenesis.32-33 Hence, a shift in the flow of mesenchymal precursor cells from osteoblastic to adipogenic lineages mediated by activation of PPAR{gamma} may result in reduced bone formation and, ultimately, bone loss. Consistently, recent studies in rodent models14, 17-18 and humans19-21 indicate that exposure to thiazolidinediones impairs osteoblastic function, resulting in reduced bone formation and bone mass. In contrast, results of studies15, 17-18,20 evaluating the short-term effects of rosiglitazone on osteoclastic bone resorption demonstrated that thiazolidinediones do not affect bone resorption in vivo. Nevertheless, uncoupling of bone formation from bone resorption was accompanied by early bone loss. These changes resemble the pattern of alteration of bone remodeling after initiation of glucocorticoid therapy, which is accompanied by rapid bone loss and increased risk of fragility fractures.34 In this study, short-term exposure did not materially alter the fracture risk, whereas fracture risk increased in current users with therapy duration of 12 months and more.
Results of clinical trial databases23-24 and findings from ADOPT22 indicated that the increased risk of fractures is limited to women. In contrast, the present findings do not necessarily support the proposition that a deleterious effect on bones may be restricted to women because in this study the fracture risk was also increased in men. Accelerated bone loss has been observed in postmenopausal women19-20 and older men21 treated with thiazolidinediones. Ultimately, randomized controlled trials that include women and men are needed to provide evidence for or against a sex difference in the skeletal response to glitazones.
This study has several limitations. First, we cannot exclude the possibility that some fractures may have been missed or misclassified. We focused on clinical osteoporotic fractures resulting from low trauma. Systematic radiographic screening to identify asymptomatic vertebral deformities and fractures is not available; consequently, the number of vertebral fractures may be underestimated. Second, it is conceivable that certain demographic or lifestyle factors, such as socioeconomic status, dietary habits, and physical activity, are associated with fracture risk and with use of antidiabetic drugs. However, a distortion could have occurred only if a confounder were a strong risk factor for fractures and at the same time associated particularly with the use of thiazolidinediones but not with other antidiabetic agents. To control for socioeconomic status, as much as possible, case patients and controls were matched on general practice. Third, although we adjusted for asthma/chronic obstructive pulmonary disease (and thereby, to some degree, for corticosteroid use) and for a history of stroke, it is conceivable that the observed fracture risk could have been the result of residual confounding by these risk factors. We, therefore, conducted a sensitivity analysis in which we excluded individuals with asthma/chronic obstructive pulmonary disease or stroke; the OR for current thiazolidinedione use of 15 or more prescriptions even increased (adjusted OR, 5.62; 95% CI, 2.33-13.60). Fourth, it is conceivable that patients treated with oral antidiabetic agents contributed different amounts of person-time than untreated patients with DM, which could lead to a distortion of the fracture risk. We, therefore, adjusted for duration of DM history, which left the results unchanged. Finally, the present finding of an increased fracture risk associated with thiazolidinedione use may be real, but it does not prove an adverse effect of thiazolidinedione on bone mass. In theory, alternative explanations for the observed association are conceivable.
In summary, this nested case-control analysis of diabetic patients provides additional evidence that the use of thiazolidinediones for approximately 12 or more months may increase the risk of osteoporotic nonvertebral fractures. No such effect was seen for other antidiabetic drugs in this study population. These findings, although they are consistent with recently reported data from a randomized trial,22 are based on relatively few thiazolidinedione-exposed patients and need to be confirmed by additional observational studies and by controlled clinical trials.
INTRODUCTION
Osteoporotic fractures are associated with significant morbidity and mortality and a reduction in quality of life. An estimated 30% to 50% of women and 15% to 30% of men will experience a fracture related to osteoporosis in their lifetime. Patients with type 2 diabetes mellitus (DM) have been shown to have higher bone density1-4 and, thus, might be expected to be at lower risk for fracture.5 In contrast, however, studies in community-dwelling individuals have shown an increased risk of fragility fractures, predominantly at nonvertebral sites,5-11 independent of age, body mass index (BMI), and bone density.12 The increased risk might, therefore, be related to other factors, such as diabetic complications, risk of falls, and, potentially, antidiabetic medication use.
The insulin-sensitizing thiazolidinediones are a relatively new and effective class of oral antidiabetic agents that have gained wide use in clinical conditions characterized by insulin resistance. Pioglitazone hydrochloride and rosiglitazone maleate account for 21% of the oral antidiabetic drugs in the United States and 5% in Europe.13 Preclinical14-18 and clinical19-21 studies indicate that thiazolidinediones may exert unfavorable effects on bone, resulting in reduced osteoblastic bone formation and accelerated bone loss. In a recent, large, randomized, clinical trial22 comparing the glycemic control of rosiglitazone relative with metformin hydrochloride or glyburide monotherapies, an increased risk of distal upper and distal lower limb fractures in women with type 2 DM treated with rosiglitazone was observed. Based on a Food and Drug Administration MedWatch safety information alert, an increased fracture risk seems to be related to the use of pioglitazone too, indicating a possible class effect of thiazolidinediones.23-24
Given that the use of rosiglitazone and pioglitazone is becoming more common, it is important to determine whether these drugs impair skeletal health and increase fracture risk. Specifically, it remains unclear whether thiazolidinedione use is associated with peripheral fracture sites only and whether this effect is sex specific. In this large population-based study, we explore the association between thiazolidinedione use and use of other oral antidiabetic drugs and the risk of fractures in women and men aged 30 to 89 years.
RESULTS
The study population consisted of 66 696 diabetic patients: 16 648 had a DM diagnosis with no treatment with oral antidiabetic agents or insulin, and 50 048 received at least 1 prescription for at least 1 study drug. The mean (SD) age of the study population was 60.7 (11.7) years, and 54.8% were women. In the study population, we identified 1020 case patients with fracture and 3728 matched controls. Clinically diagnosed low-trauma fractures consisted of 301 wrist/forearm, 274 hip, 222 humerus, 148 rib, 56 vertebral, and 19 other, unspecified fractures.
The age and sex distribution and the prevalence of relevant comorbidities of case patients and controls, together with crude and adjusted independent ORs, are given in Table 1. Of the 1020 case patients with fracture and their 3728 controls, 208 and 762, respectively, did not use any oral antidiabetic drugs or insulin. Of the remaining 812 case patients who used antidiabetic drugs, 65 used thiazolidinediones, all in combination with other oral antidiabetic drugs, and 747 used other oral antidiabetic agents. The ORs of developing a fracture in association with the use of 1 to 7, 8 to 14, and 15 or more thiazolidinedione prescriptions, regardless of timing of use, compared with nonuse of thiazolidinediones and adjusted for the use of other antidiabetic agents, smoking, BMI, comorbidities, and comedication, were 0.93 (95% CI, 0.57-1.52), 1.55 (95% CI, 0.81-2.94), and 2.07 (95% CI, 1.21-3.56), respectively. When we combined duration of use with timing into 1 variable, the adjusted OR of any fracture for current use of 15 or more thiazolidinedione prescriptions was 2.86 (95% CI, 1.57-5.22) (Table 2).
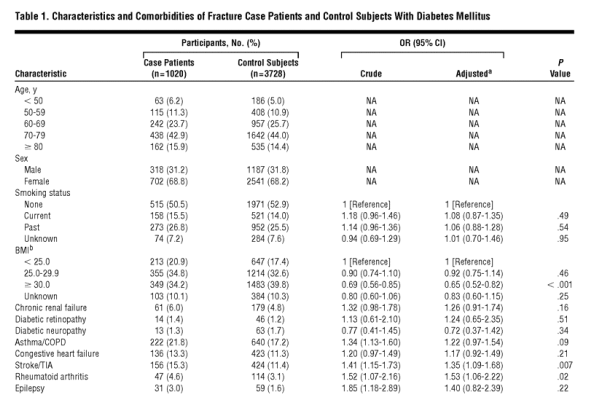
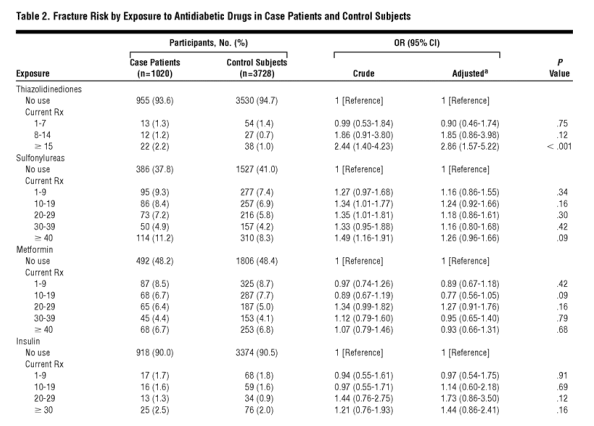
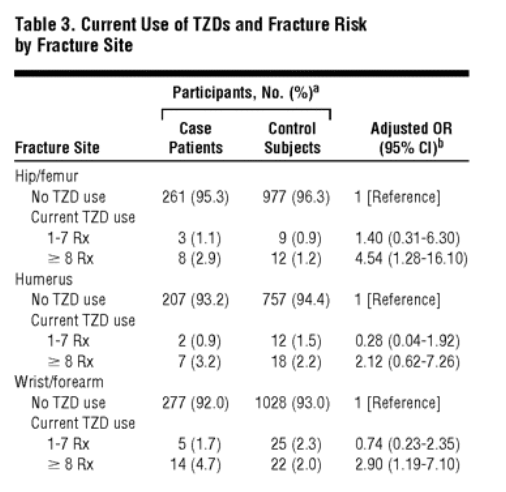
To gain statistical power and because the risk estimates were similar for users of 8 to 14 and 15 or more prescriptions, we combined current users of 8 to 14 or of 15 or more prescriptions into 1 group for further analyses; the adjusted OR for current use of 8 or more thiazolidinedione prescriptions compared with nonuse was 2.43 (95% CI, 1.49-3.95). We then stratified the group of current users of 8 or more thiazolidinedione prescriptions by sex and age, yielding adjusted ORs of 2.50 (95% CI, 0.84-7.41) for men, 2.56 (95% CI, 1.43-4.58) for women, 2.96 (95% CI, 1.40-6.25) for patients younger than 70 years, and 2.57 (95% CI, 1.22-5.40) for those 70 years and older. We also stratified thiazolidinedione users by individual thiazolidinedione and by tablet dose. Current use of 8 or more prescriptions for pioglitazone or rosiglitazone yielded adjusted ORs of 2.59 (95% CI, 0.96-7.01) and 2.38 (95% CI, 1.39-4.09), respectively. The adjusted risk estimates for current use of 8 or more prescriptions of a low dose (4 mg of rosiglitazone maleate or 15 mg of pioglitazone hydrochloride) or of a high dose (8 mg of rosiglitazone maleate or 30 mg of pioglitazone hydrochloride) were 2.13 (95% CI, 1.15-3.92) and 2.98 (95% CI, 1.42-6.26), respectively. Adjusted risk estimates stratified by fracture site are given in Table 3.
METHODS
DATA SOURCE
Data were derived from the UK-based General Practice Research Database (GPRD), which has been described previously.25-26 Briefly, this database was established around 1987 and currently encompasses approximately 5 million people who are enrolled with selected general practitioners, covering more than 50 million patient-years of follow-up. The patients enrolled in the GPRD are representative of the United Kingdom regarding age, sex, geographic distribution, and annual turnover rate. General practitioners have been trained to record medical information, including demographic data, medical diagnoses, hospitalizations, deaths, and drug prescriptions, using standard software and standard coding systems. The general practitioners generate prescriptions directly using the computer, and this information is automatically transcribed into the computer record. It contains the name of the preparation, route of administration, dose, and number of tablets for each prescription. The recorded information on drug exposure and diagnoses has been validated and proved to be of high quality.27-28 The GPRD has been the source of many observational studies, including research on fractures.29-30 Based on previous medical record reviews,29, 31 we determined that the validity of fracture diagnoses in the GPRD is high, with a confirmed proportion of at least 90% after comparing computer-recorded diagnoses with hospital discharge letters or questionnaire information provided by general practitioners. The study was approved by the Independent Scientific Advisory Committee for Medicines and Healthcare Products Regulatory Agency database research.
STUDY POPULATION
We identified in the GPRD all individuals who received at least 1 prescription for a thiazolidinedione (pioglitazone, rosiglitazone, or troglitazone), a sulfonylurea derivative (glyburide, gliclazide, glipizide, glimepiride, glibornuride, gliquidone, tolbutamide, chlorpropamide, tolazamide, or acetohexamide), the biguanide metformin, the {alpha}-glucosidase inhibitor acarbose, or a prandial glucose regulator (repaglinide or nateglinide), with or without concomitant insulin use, between ages 30 and 79 years and between January 1994 and December 2005. In addition, we identified all individuals with a recorded DM diagnosis between ages 30 and 79 years (at the first DM recording) who never received a prescription for an oral antidiabetic drug or insulin. We did not include patients with type 1 DM who never used oral antidiabetic agents. We excluded all patients with less than 3 years of recorded history in the database before the first prescription for an antidiabetic drug or the first DM diagnosis, whichever came first, and all patients with a diagnosis of alcoholism, a history of cancer (except nonmelanoma skin cancer), or a diagnosis of gestational DM at any time in their medical record.
CASE PATIENT DEFINITION AND CONTROL SUBJECT SELECTION
In this study population of diabetic patients, we identified all patients with a first-time diagnosis of a low-trauma fracture (including vertebral fractures and nonvertebral fractures of the hip, wrist, proximal humerus, and ribs) after the first DM diagnosis or after the first prescription for an oral antidiabetic drug, whichever came first, between ages 30 and 89 years. The fracture date is referred to as the "index date." We excluded patients with a diagnosis of Paget disease, osteoporosis, or osteomalacia and patients who used bisphosphonates (as an indicator of osteoporosis or bone metastases) before the index date.
In the same study population, we identified at random up to 4 control subjects per case patient (ie, subjects who did not have a fracture diagnosis recorded), matched to case patients on age (same year of birth), sex, general practice attended, and index date (ie, the date of the fracture diagnosis of the case patient). The same exclusion criteria were applied to controls as to case patients.
STATISTICAL ANALYSIS
We assessed, from the computer records, exposure to oral antidiabetic agents and insulin before the index date in case patients with fracture and controls. We classified users of oral antidiabetic drugs according to drug class (thiazolidinediones, sulfonylureas, metformin, prandial glucose regulators, or {alpha}-glucosidase inhibitors), timing of exposure ("current use" if the last prescription for a drug of interest was recorded < 60 days or "past use" if it was recorded ≥ 60 days before the index date), and duration of use, based on the number of prescriptions before the index date; we classified duration of thiazolidinedione use into categories of 1 to 7, 8 to 14, or 15 or more prescriptions. For insulin, prandial glucose regulators, and acarbose, categories were defined as use of 1 to 9, 10 to 19, 20 to 29, or 30 or more prescriptions; and for sulfonylureas and metformin (owing to a higher exposure prevalence of these drugs), groups were divided into use of 1 to 9, 10 to 19, 20 to 29, 30 to 39, or 40 or more prescriptions.
We conducted conditional logistic regression analyses using a software program (SAS 8.1; SAS Institute Inc, Cary, North Carolina). Risk estimates are presented as odds ratios (ORs) with 95% confidence intervals (CIs). The P values are 2-sided, and P < .05 was considered statistically significant. We compared thiazolidinedione use with nonuse of thiazolidinediones and adjusted for use of other antidiabetic agents and for smoking status (none, current, past, or unknown); BMI (calculated as weight in kilograms divided by height in meters squared) (< 25, 25-29.9, and ≥ 30); a variety of diagnosed comorbidities potentially associated with an altered fracture risk, such as chronic renal failure, diabetic neuropathy, diabetic retinopathy, asthma/chronic obstructive pulmonary disease, congestive heart failure, a history of stroke/transient ischemic attack, epilepsy, or rheumatoid arthritis; and use of antihypertensive drugs, diuretics, lipid-lowering agents, inhaled or systemic corticosteroids, antiepileptic drugs, benzodiazepines, antipsychotic agents, or antidepressants in the multivariate model. We further assessed the duration of DM history, defined as the period between the first DM diagnosis or the first prescription for an oral antidiabetic drug and the index date.
REFERENCES
1. Isaia GC, Ardissone P, Di Stefano M; et al. Bone metabolism in type 2 diabetes mellitus. Acta Diabetol. 1999;36(1-2):35-38. FULL TEXT ISI PUBMED
2. Barrett-Connor E, Holbrook TL. Sex differences in osteoporosis in older adults with non-insulin-dependent diabetes mellitus. JAMA. 1992;268(23):3333-3337. ABSTRACT
3. van Daele PL, Stolk RP, Burger H; et al. Bone density in non-insulin-dependent diabetes mellitus: the Rotterdam Study. Ann Intern Med. 1995;122(6):409-414. FREE FULL TEXT
4. Schwartz AV, Hillier TA, Sellmeyer DE; et al. Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25(10):1749-1754. FREE FULL TEXT
5. Bonds DE, Larson JC, Schwartz AV; et al. Risk of fracture in women with type 2 diabetes: the Women's Health Initiative Observational Study. J Clin Endocrinol Metab. 2006;91(9):3404-3410. FREE FULL TEXT
6. Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24(7):1192-1197. FREE FULL TEXT
7. Taylor BC, Schreiner PJ, Stone KL; et al. Long-term prediction of incident hip fracture risk in elderly white women: study of osteoporotic fractures. J Am Geriatr Soc. 2004;52(9):1479-1486. FULL TEXT ISI PUBMED
8. Strotmeyer ES, Cauley JA, Schwartz AV; et al. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2004;19(7):1084-1091. FULL TEXT ISI PUBMED
9. Strotmeyer ES, Cauley JA, Schwartz AV; et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the Health, Aging, and Body Composition Study. Arch Intern Med. 2005;165(14):1612-1617. FREE FULL TEXT
10. de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: the
Rotterdam Study. Osteoporos Int. 2005;16(12):1713-1720. FULL TEXT ISI PUBMED
11. Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48(7):1292-1299. FULL TEXT ISI PUBMED
12. Schwartz AV, Sellmeyer DE, Ensrud KE; et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32-38. FREE FULL TEXT
13. Yki-Jarvinen H. The PROactive study: some answers, many questions. Lancet. 2005;366(9493):1241-1242. FULL TEXT ISI PUBMED
14. Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401-406. FREE FULL TEXT
15. Soroceanu MA, Miao D, Bai XY, Su H, Goltzman D, Karaplis AC. Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. J Endocrinol. 2004;183(1):203-216. FREE FULL TEXT
16. Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPAR{gamma} agonist BRL49653 (rosiglitazone). Calcif Tissue Int. 2004;75(4):329-337. FULL TEXT ISI PUBMED
17. Akune T, Ohba S, Kamekura S; et al. PPAR{gamma} insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113(6):846-855. FULL TEXT ISI PUBMED
18. Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146(3):1226-1235. FREE FULL TEXT
19. Schwartz AV, Sellmeyer DE, Vittinghoff E; et al. Thiazolidinedione use and bone loss in older diabetic adults. J Clin Endocrinol Metab. 2006;91(9):3349-3354. FREE FULL TEXT
20. Grey A, Bolland M, Gamble G; et al. The peroxisome proliferator-activated receptor-{gamma} agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92(4):1305-1310. FREE FULL TEXT
21. Yaturu S, Bryant B, Jain SK. Thiazolidinediones treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care. 2007;30(6):1574-1576. FREE FULL TEXT
22. Kahn SE, Haffner SM, Heise MA; et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427-2443. FREE FULL TEXT
23. Short R. Fracture risk is a class effect of glitazones [news]. BMJ. 2007;334(7593):551. FULL TEXT ISI PUBMED
24. Hampton T. Diabetes drugs tied to fractures in women [news]. JAMA. 2007;297(15):1645. FREE FULL TEXT
25. Garcia Rodriguez LA, Perez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45(5):419-425. FULL TEXT ISI PUBMED
26. Wood L, Martinez C. The general practice research database: role in pharmacovigilance. Drug Saf. 2004;27(12):871-881. FULL TEXT ISI PUBMED
27. Jick H, Jick SS, Derby LE. Validation of information recorded on general practitioner based computerised data resource in the United Kingdom. BMJ. 1991;302(6779):766-768. ISI PUBMED
28. Jick SS, Kaye JA, Vasilakis-Scaramozza C; et al. Validity of the general practice research database. Pharmacotherapy. 2003;23(5):686-689. FULL TEXT ISI PUBMED
29. Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA. 2000;283(24):3205-3210. FREE FULL TEXT
30. Schlienger RG, Kraenzlin ME, Jick SS, Meier CR. Use of β-blockers and risk of fractures. JAMA. 2004;292(11):1326-1332. FREE FULL TEXT
31. van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford). 2000;39(12):1383-1389. FREE FULL TEXT
32. Lazarenko OP, Rzonca SO, Hogue WR, Swain FL, Suva LJ, Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148(6):2669-2680. FREE FULL TEXT
33. Lecka-Czernik B, Gubrij I, Moerman EJ; et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPAR{gamma}2. J Cell Biochem. 1999;74(3):357-371. FULL TEXT ISI PUBMED
34. Ton FN, Gunawardene SC, Lee H, Neer RM. Effects of low-dose prednisone on bone metabolism. J Bone Miner Res. 2005;20(3):464-470. FULL TEXT ISI PUBMED
|
|
| |
| |
|
|
|