| |
Detection of Nonnucleoside Reverse-Transcriptase Inhibitor-Resistant HIV-1 after Discontinuation (Interruption) of Virologically Suppressive Antiretroviral Therapy
|
| |
| |
Clinical Infectious Diseases Aug 1, 2008;47:421-424
C. Bradley Hare,1 John Mellors,2 Amy Krambrink,3 Zhaohui Su,3 Daniel Skiest,4 David M. Margolis,5 Sheran S. Patel,2 Douglas Barnas,2 Lisa Frenkel,6 Robert W. Coombs,6 Francesca Aweeka,1 Gene D. Morse,7 David W. Haas,8 Valerie Boltz,9 Sarah Palmer,9 John Coffin,9 and Diane V. Havlir1
1University of California, San Francisco; 2University of Pittsburgh, Pittsburgh, Pennsylvania; 3Statistical and Data Analysis Center/Harvard School of Public Health, Boston, and 4Baystate Medical Center, Springfield, Massachusetts; 5University of North Carolina, Chapel Hill; 6University of Washington, Seattle; 7University at Buffalo, State University of New York at Buffalo; 8Vanderbilt University, Nashville, Tennessee; and 9HIV Drug Resistance Program, National Cancer Institute at Frederick, Maryland
"......we observed that individuals who discontinue nonnucleoside reverse-transcriptase inhibitor-containing antiretroviral therapy when their plasma HIV-1 RNA level is <400 copies/mL are at substantial risk (20%) of virologic rebound with a nonnucleoside reverse-transcriptase inhibitor-resistant virus. Allele-specific PCR detected more resistance mutations in these subjects. This assay has also been shown to detect more transmitted HIV-1 drug resistance, which was associated with higher rates of virologic failure......In our study, the risk of resistance was greater for those who discontinued therapy when HIV-1 RNA level was 51-400 copies/mL than when it was 50 copies/mL. This viral load may represent either intermittent or sustained low-level replication. Brief periods of intermittent low-level viral load, or "blips," have previously been shown not to correlate with virologic failure [8], whereas sustained low-level replication during therapy may result in the evolution of viral resistance and virologic failure [9, 10]. Thus, low-level viral replication may permit selection of mutants that remain largely controlled during continued combination drug therapy but may become detectable among circulating variants once therapy is interrupted.....other studies indicate that brief interruptions in therapy for as many as 7 days did not seem to be associated with drug resistance [12, 13]. Moreover, in our study, discontinuation of nonnucleoside reverse-transcriptase inhibitors 2 days before the other components of the antiretroviral regimen did not completely protect against resistance. In aggregate, these observations suggest that prolonged interruption or discontinuation of nonnucleoside reverse-transcriptase inhibitor regimens during periods of low-level viral load should be avoided.....An observation of clinical significance in our study is the persistence of detectable drug-resistant virus selected by therapy interruption....When nonnucleoside reverse-transcriptase inhibitor resistance was detected by population sequencing at virologic rebound, the K103N mutation could be detected in most subjects throughout follow-up while they were not undergoing antiretroviral therapy. This finding suggests that genotype testing may be clinically useful for 1 year or more among subjects who have discontinued adherence to a nonnucleoside reverse-transcriptase inhibitor-containing regimen.....subjects in this study had high CD4+ T cell counts and asymptomatic HIV disease. Caution must be used when generalizing our results to different populations....These results underscore the importance of developing strategies that minimize resistance during planned interruptions of nonnucleoside reverse-transcriptase inhibitor-containing antiretroviral therapy. For unplanned interruptions, genotyping may be useful to guide subsequent therapy...."
ABSTRACT: Using standard and ultrasensitive techniques, we detected nonnucleoside reverse-transcriptase inhibitor-associated resistance mutations in 11 (20%) of 54 subjects who discontinued virologically suppressive nonnucleoside reverse-transcriptase inhibitor-containing antiretroviral therapy. Resistance was detected in 45% and 14% of subjects with a baseline human immunodeficiency virus type 1 RNA level of 51-400 copies/mL and 50 copies/mL, respectively. Mutations remained detectable for at least 48 weeks in some subjects.
RESULTS: Mutations associated with nonnucleoside reverse-transcriptase inhibitor resistance were detected in 11 (20%) of 54 subjects at the time of virologic rebound. In 5 subjects, mutations were detected by population sequencing, whereas an additional 6 subjects had mutations detected only by allele-specific PCR, occurring at frequencies well below the limit of detection of the population-sequencing method. Six subjects had mutations detected by oligonucleotide ligation assay before antiretroviral therapy discontinuation, 3 of whom also had mutations detected at virologic rebound; 1 of these mutations was at a different codon. Table 1 summarizes all the nonnucleoside reverse-transcriptase inhibitor mutations detected.
Five subjects had K103N mutations detected by population sequencing at the time of virologic rebound. One subject resumed antiretroviral therapy within 4 weeks of discontinuation. Three of the 4 remaining subjects had K103N detected by population sequencing throughout 36-48 weeks of treatment interruption.
In multivariable analyses, both an HIV-1 RNA level of 51-400 copies/mL (OR, 4.9; 95% CI, 1.0-25.4; p=.05 ) and subtherapeutic nonnucleoside reverse-transcriptase inhibitor concentrations (OR, 6.0; 95% CI, 1.2-32.4; p=.03) were predictive of resistance by either assay at the time of virologic rebound. Five (45%) of 11 subjects who discontinued antiretroviral therapy with an HIV-1 RNA level of 51-400 copies/mL displayed detectable resistance mutations, compared with 6 (14%) of 43 subjects with an HIV-1 RNA level of <50 copies/mL. Five (50%) of 10 subjects who discontinued antiretroviral therapy with subtherapeutic nonnucleoside reverse-transcriptase inhibitor drug concentrations demonstrated detectable resistance mutations, compared with 6 (14%) of 42 subjects with therapeutic nonnucleoside reverse-transcriptase inhibitor drug concentrations.
INTRODUCTION: Antiretroviral therapy has significantly reduced morbidity and mortality from HIV-1 infection; however, prolonged adherence to antiretroviral therapy can be challenging, and treatment interruptions are common, even among subjects who have achieved virologic suppression [1]. Interruptions of antiretroviral therapy may facilitate the emergence of drug-resistant HIV-1 by exposing actively replicating virus to selective pressure from 1 or more antiretroviral agents. Interruptions to regimens that include the nonnucleoside reverse-transcriptase inhibitors efavirenz or nevirapine pose a particular risk of selecting for drug-resistant HIV-1 because of the long half-life and low genetic barrier to resistance of these drugs. In this study, we used both population sequencing and a more sensitive allele-specific PCR technique to examine the frequency, timing, and predictors of nonnucleoside reverse-transcriptase inhibitor resistance among individuals undergoing antiretroviral therapy who discontinue virologically suppressive regimens.
BRIEF REPORT
Detection of Nonnucleoside Reverse-Transcriptase Inhibitor-Resistant HIV-1 after Discontinuation of Virologically Suppressive Antiretroviral Therapy
C. Bradley Hare,1 John Mellors,2 Amy Krambrink,3 Zhaohui Su,3 Daniel Skiest,4David M. Margolis,5 Sheran S. Patel,2 Douglas Barnas,2 Lisa Frenkel,6 Robert W. Coombs,6Francesca Aweeka,1 Gene D. Morse,7 David W. Haas,8 Valerie Boltz,9 Sarah Palmer,9 John Coffin,9and Diane V. Havlir1
1University of California, San Francisco; 2University of Pittsburgh, Pittsburgh, Pennsylvania;3Statistical and Data Analysis Center/Harvard School of Public Health, Boston, and 4Baystate Medical Center, Springfield, Massachusetts; 5University of North Carolina, Chapel Hill; 6University of Washington, Seattle; 7University at Buffalo, State University of New York at Buffalo;8Vanderbilt University, Nashville, Tennessee; and 9HIV Drug Resistance Program, National Cancer Institute at Frederick, Maryland
ABSTRACT
Using standard and ultrasensitive techniques, we detected nonnucleoside reverse-transcriptase inhibitor-associated resistance mutations in 11 (20%) of 54 subjects who discontinued virologically suppressive nonnucleoside reverse-transcriptase inhibitor-containing antiretroviral therapy. Resistance was detected in 45% and 14% of subjects with a baseline human immunodeficiency virus type 1 RNA level of 51-400 copies/mL and <50 copies/mL, respectively. Mutations remained detectable for at least 48 weeks in some subjects.
Antiretroviral therapy has significantly reduced morbidity and mortality from HIV-1 infection; however, prolonged adherence to antiretroviral therapy can be challenging, and treatment interruptions are common, even among subjects who have achieved virologic suppression [1]. Interruptions of antiretroviral therapy may facilitate the emergence of drug-resistant HIV-1 by exposing actively replicating virus to selective pressure from 1 or more antiretroviral agents. Interruptions to regimens that include the nonnucleoside reverse-transcriptase inhibitors efavirenz or nevirapine pose a particular risk of selecting for drug-resistant HIV-1 because of the long half-life and low genetic barrier to resistance of these drugs. In this study, we used both population sequencing and a more sensitive allele-specific PCR technique to examine the frequency, timing, and predictors of nonnucleoside reverse-transcriptase inhibitor resistance among individuals undergoing antiretroviral therapy who discontinue virologically suppressive regimens.
Methods
Subjects participated in the AIDS Clinical Trials Group protocol A5170, a prospective, 2-year, observational study of antiretroviral therapy discontinuation among individuals with asymptomatic HIV-1 infection and a CD4+ T cell count nadir >350 cells/mm3, the primary results of which are reported elsewhere [2]. This analysis examined the subset of participants who were receiving nonnucleoside reverse-transcriptase inhibitor-containing regimens and had a plasma HIV-1 RNA level <400 copies/mL at the time of antiretroviral therapy discontinuation. Protocol A5170 required discontinuation of nonnucleoside reverse-transcriptase inhibitor therapy at study entry and recommended discontinuing the use of other antiretrovirals 2 days later.
Plasma was assayed at baseline and every 4 weeks after treatment interruption for HIV-1 RNA quantification with use of the Ultrasensitive Roche Amplicor HIV-1 Monitor assay (Roche Molecular Diagnostics), with a limit of quantification of 50 copies/mL. At the time of the first virologic rebound to a plasma HIV-1 RNA level >5000 copies/mL after treatment interruption, samples were assayed for drug resistance mutations. Informed consent was obtained from all subjects involved in protocol A5170, and human experimentation guidelines of the US Department of Health and Human Services were followed.
Standard population sequencing was performed on all samples at the time of virologic rebound, with use of the ViroSeq v2.6 assay (Applied Biosystems). When mutations were detected by population sequencing, repeat sequencing was performed every 4-12 weeks until the end of the study or until subjects chose to reinitiate antiretroviral therapy.
If no resistance mutations were detected by population sequencing at the time of virologic rebound, allele-specific PCR was performed to test for low-level mutant variants. We performed allele-specific PCR assays for mutations at reverse-transcriptase codons 103 and 181, as described elsewhere, with a limit of detection of mutant virus of 0.1% [3].
To test for the presence of nonnucleoside reverse-transcriptase inhibitor mutations that existed before antiretroviral therapy discontinuation, peripheral blood mononuclear cells from baseline were tested for K103N, Y181C, and G190A with use of the oligonucleotide ligation assay, according to the method detailed elsewhere [4].
High-performance liquid chromatography was used to quantify efavirenz and nevirapine concentrations in plasma at the time of discontinuation of nonnucleoside reverse-transcriptase inhibitor therapy and every 4 weeks thereafter until levels were below the limits of detection (50 ng/mL for efavirenz and 200 ng/mL for nevirapine). We considered therapeutic concentrations of efavirenz and nevirapine to be 1-4 _g/mL and 3-12 _g/mL, respectively [5, 6].
A descriptive statistical analysis of subject characteristics was performed at study entry. Wilcoxon exact rank sum tests were used to compare the time to first detection of an HIV-1 RNA level >5000 copies/mL between subjects with and without mutations. Logistic regression analysis with use of the profile-likelihood approach was used to examine predictors of resistance mutations detected at virologic rebound. Fisher's exact test was were used to verify the P values from univariate analyses. Variables with p<.10 from univariate regression analyses were included in multivariable analyses. All tests were 2-sided, and p<.05 was considered to be statistically significant. Statistical analyses were performed using SAS statistical software, version 9.1 (SAS).
Results
The mean age of the 54 study subjects was 43 years; 85% (46 subjects) were men. The mean HIV-1 RNA level before antiretroviral therapy initiation was 4.6 log copies/mL. Subjects had received antiretroviral therapy a mean of 32 months before treatment discontinuation. Thirty-one subjects were undergoing their initial regimen; 23 had undergone successive antiretroviral therapy regimens. One subject was taking efavirenz with a protease inhibitor alone; all other subjects had nucleoside reverse-transcriptase inhibitors as part of their regimens. Four additional subjects also included a protease inhibitor (3 were receiving nevirapine and 1 was receiving efavirenz). Among the 33 subjects receiving efavirenz at the time of treatment discontinuation, 27 had therapeutic plasma levels, 4 had subtherapeutic levels, and data for 2 were missing. Among the 21 subjects receiving nevirapine, 15 had therapeutic plasma levels and 6 had subtherapeutic levels. Forty-three subjects discontinued antiretroviral therapy with an HIV-1 RNA level of 50 copies/mL; 11 discontinued with an HIV-1 RNA level of 51-400 copies/mL.
Twenty-two participants (41%) experienced virologic rebound to an HIV-1 RNA level >5000 copies/mL by 4 weeks after treatment discontinuation, 46 participants (85%) experienced virologic rebound by 8 weeks, and all participants experienced virologic rebound by 60 weeks. No association was found between the time to virologic rebound and detection of resistance mutations (p=.40, by Wilcoxon exact rank sum test).
Mutations associated with nonnucleoside reverse-transcriptase inhibitor resistance were detected in 11 (20%) of 54 subjects at the time of virologic rebound. In 5 subjects, mutations were detected by population sequencing, whereas an additional 6 subjects had mutations detected only by allele-specific PCR, occurring at frequencies well below the limit of detection of the population-sequencing method. Six subjects had mutations detected by oligonucleotide ligation assay before antiretroviral therapy discontinuation, 3 of whom also had mutations detected at virologic rebound; 1 of these mutations was at a different codon. Table 1 summarizes all the nonnucleoside reverse-transcriptase inhibitor mutations detected.
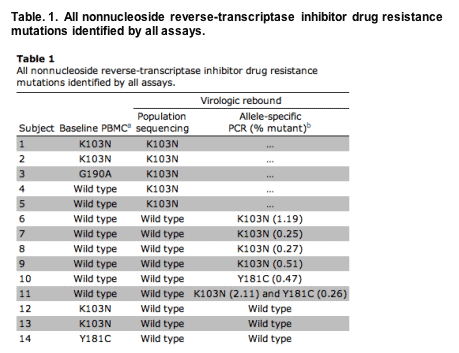
Five subjects had K103N mutations detected by population sequencing at the time of virologic rebound. One subject resumed antiretroviral therapy within 4 weeks of discontinuation. Three of the 4 remaining subjects had K103N detected by population sequencing throughout 36-48 weeks of treatment interruption.
In multivariable analyses, both an HIV-1 RNA level of 51-400 copies/mL (OR, 4.9; 95% CI, 1.0-25.4; p=.05) and subtherapeutic nonnucleoside reverse-transcriptase inhibitor concentrations (OR, 6.0; 95% CI, 1.2-32.4; p=.03 ) were predictive of resistance by either assay at the time of virologic rebound. Five (45%) of 11 subjects who discontinued antiretroviral therapy with an HIV-1 RNA level of 51-400 copies/mL displayed detectable resistance mutations, compared with 6 (14%) of 43 subjects with an HIV-1 RNA level of 50 copies/mL. Five (50%) of 10 subjects who discontinued antiretroviral therapy with subtherapeutic nonnucleoside reverse-transcriptase inhibitor drug concentrations demonstrated detectable resistance mutations, compared with 6 (14%) of 42 subjects with therapeutic nonnucleoside reverse-transcriptase inhibitor drug concentrations.
Discussion
In this study, we observed that individuals who discontinue nonnucleoside reverse-transcriptase inhibitor-containing antiretroviral therapy when their plasma HIV-1 RNA level is <400 copies/mL are at substantial risk (20%) of virologic rebound with a nonnucleoside reverse-transcriptase inhibitor-resistant virus. Allele-specific PCR detected more resistance mutations in these subjects. This assay has also been shown to detect more transmitted HIV-1 drug resistance, which was associated with higher rates of virologic failure [7].
In our study, the risk of resistance was greater for those who discontinued therapy when HIV-1 RNA level was 51-400 copies/mL than when it was 50 copies/mL. This viral load may represent either intermittent or sustained low-level replication. Brief periods of intermittent low-level viral load, or "blips," have previously been shown not to correlate with virologic failure [8], whereas sustained low-level replication during therapy may result in the evolution of viral resistance and virologic failure [9, 10]. Thus, low-level viral replication may permit selection of mutants that remain largely controlled during continued combination drug therapy but may become detectable among circulating variants once therapy is interrupted. This model is consistent with a prior study of structured intermittent therapy, which was terminated early when 3 of 8 patients adhering to an efavirenz-containing regimen demonstrated newly emergent resistance mutations [11]. In contrast, other studies indicate that brief interruptions in therapy for as many as 7 days did not seem to be associated with drug resistance [12, 13]. Moreover, in our study, discontinuation of nonnucleoside reverse-transcriptase inhibitors 2 days before the other components of the antiretroviral regimen did not completely protect against resistance. In aggregate, these observations suggest that prolonged interruption or discontinuation of nonnucleoside reverse-transcriptase inhibitor regimens during periods of low-level viral load should be avoided.
An observation of clinical significance in our study is the persistence of detectable drug-resistant virus selected by therapy interruption. Nonnucleoside reverse-transcriptase inhibitor resistance mutations have been demonstrated to persist in plasma for years, as noted in the prevention of mother-to-child transmission [14], transmitted drug resistance [15], failure of nonnucleoside reverse-transcriptase inhibitor-containing therapy [3], and CD4-guided structured treatment interruption [16]. When nonnucleoside reverse-transcriptase inhibitor resistance was detected by population sequencing at virologic rebound, the K103N mutation could be detected in most subjects throughout follow-up while they were not undergoing antiretroviral therapy. This finding suggests that genotype testing may be clinically useful for 1 year or more among subjects who have discontinued adherence to a nonnucleoside reverse-transcriptase inhibitor-containing regimen.
Our study had several limitations. First, we did not have plasma HIV-1 RNA specimens before the original initiation of antiretroviral therapy and cannot exclude the possibility that mutations predated treatment. However, sustained virologic suppression of these individuals who adhere to nonnucleoside reverse-transcriptase inhibitor-based regimens argues against this possibility. Second, allele-specific PCR detected only prespecified mutations at positions 103, 181, and 190; mutations at other positions may have been present and undetected. Third, detailed nonnucleoside reverse-transcriptase inhibitor pharmacokinetic analyses were limited by the availability of samples from only the time of treatment discontinuation and 4 weeks later, by which time all subjects had undetectable nonnucleoside reverse-transcriptase inhibitor concentrations. Analysis of data on 23 subjects who resumed therapy during study observation was limited by small numbers and the heterogeneous nature of subsequent regimens. Further studies are warranted in this important area. Finally, subjects in this study had high CD4+ T cell counts and asymptomatic HIV disease. Caution must be used when generalizing our results to different populations.
These results underscore the importance of developing strategies that minimize resistance during planned interruptions of nonnucleoside reverse-transcriptase inhibitor-containing antiretroviral therapy. For unplanned interruptions, genotyping may be useful to guide subsequent therapy.
|
|
| |
| |
|
|
|