| |
The paradoxical effects of using antiretroviral-based microbicides to control HIV epidemics: risk for HIV drug resistance
|
| |
| |
PNAS July 15, 2008
David P. Wilson*, Paul M. Coplan, Mark A. Wainberg, and Sally M. Blower
*National Centre in HIV Epidemiology and Clinical Research, Faculty of Medicine, University of New South Wales, Level 2, 376 Victoria Street, Sydney NSW
2010, Australia; Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania School of Medicine, 423 Guardian Drive, Philadelphia, PA
19104; McGill University AIDS Centre, Jewish General Hospital, 3755 Cote-Ste.-Catherine Road, Montreal, QC, Canada H3T 1E2; and Semel Institute for
Neuroscience and Human Behavior and UCLA AIDS Institute, David Geffen School of Medicine, University of California, Los Angeles, CA 90095
"If high-risk microbicides are used in public health interventions they could substantially increase levels of ARV resistance in a population (Table 1; predictions are from our epidemiological model simulations 10 years after an intervention has been initiated). Prevalence of resistance would be greatest in women (22% median; IQR 8-50%), but transmitted resistance would be about 12 times greater in men (2.6% median; IQR 0.8-7.0%) than in women (Table 1). However, if high-risk microbicides are even moderately efficacious (about 50%) they could be beneficial in reducing the HIV pandemic by decreasing transmission (Table 1). Surprisingly, our predictions show that both high-risk and low-risk microbicides could lead to greater reductions in incidence in men than in women (Table 1 and Fig. S7). In 74% of our simulations of high-risk microbicides and in 57% of our simulations of low-risk microbicides the cumulative number of infections prevented per resistant case was also greatest in men (Fig. S9). The black line in Fig. S7 shows the point at which the cumulative number of infections prevented is equally reduced for both men and women; the blue region to the right of the line indicates when microbicides would be more beneficial for men than women. These results show that, paradoxically, although vaginal microbicides are a female-initiated prevention tool designed to protect women against HIV infection, they could often prevent more infections in men. This phenomenon is more likely to occur if high-risk microbicides are used.....
.....We have also shown that it is possible that high levels of resistance could arise when high-risk microbicides are used as public health interventions and wide scale testing does not occur. Hence, monthly testing decreases a participant's risk of resistance during the trial but increases the risk of resistance for the general population when microbicides are used as interventions and very frequent testing does not occur. We recommend that it is essential that the degree of systemic absorption of the NNRTIs and the time for resistance to emerge should be measured in phase III efficacy trials. These factors should be measured in every trial because different NNRTIs are likely to be absorbed from microbicidal gels at different rates, and they should be well characterized before marketing authorization. It is also possible, but unlikely, that selection for resistance could occur in the vaginal cavity, and that systemic absorption would not be necessary. ARV-based microbicides, like any therapeutic product, may cause other adverse events apart from resistance that should also be monitored. They should also be evaluated during clinical trials to determine whether they show differential effectiveness against different HIV subtypes (29)...... Public health interventions based on PrEP could have similar population-level consequences for resistance as ARV-based microbicides and may also have some similar gender-specific differences. Important issues for both protection options include the responsibility to prevent secondary spread of drug-resistant infection and the need for frequent medical monitoring..... the potential combined benefit of male circumcision, and/or condoms, together with the usage of microbicides could substantially reduce rates of heterosexual transmission. However, ARV-based microbicides could lead to relatively high levels of ARV resistance arising. Therefore, we recommend that careful and regular population-level monitoring occur to monitor for resistance if, and when, ARV-based microbicides are used in public health interventions. Our results imply that monitoring for resistance should occur when PrEP is widely used.... However, it is important to note that if resistance does emerge as a result of either ARV-based microbicides or PrEP, the strains that will evolve will only be resistant to the specific class of drugs in the products (3, 32). Susceptibility to other classes of ARVs will be unaffected, and hence therapeutic options for the individuals who acquire resistance will be reduced but not eliminated. In addition, recently approved secondgeneration NNRTI drugs retain efficacy against most HIV variants that are resistant to first-generation NNRTI drugs (33, 34). Other ARV-based microbicides could use different classes of drugs such as entry inhibitors. CCR5 antagonists, such as the Rantes derivatives, have the potential to be highly cost-effective and less likely to select for resistance (35, 36)....Obviously, it is advantageous for microbicides to prevent infections in both men and women. However, vaginal microbicides are being developed for preventing infections in women and to empower women, particularly in resource-constrained countries. They are a prevention tool that can only be used by women. Nevertheless, we have found that ARV-based microbicides may, under certain conditions, benefit men more than women. Whether this will occur will be determined by (i) the degree to which women adhere to microbicides, (ii) microbicide efficacy, and (iii) the viral fitness of drug-resistant strains. It is paradoxical that men may benefit more than women because of the development of effective microbicides; this paradoxical effect will be exacerbated if high-risk microbicides are used. We predict, therefore, that the population-level consequences of public health interventions that use ARV-based microbicides could well be surprising.""
Vaginal microbicides are being designed to prevent HIV infection of immune system cells and cervicovaginal tissue (1-3). They are currently considered to be one of the most promising biomedical interventions for preventing heterosexual transmission. Because the majority of the 3 million HIV infections that occur each year are in women (4, 5), effective female-initiated prevention methods are urgently needed. Second-generation microbicides, containing antiretrovirals (ARVs), are now being tested for efficacy in phase III clinical trials [supporting information (SI) Table S1]. A microbicide gel containing tenofovir, a nucleotide reverse transcriptase inhibitor, will be the first to be tested for efficacy. This will
be closely followed by a microbicide containing dapivirine, which belongs to the nonnucleoside reverse transcriptase inhibitor (NNRTI) class of ARVs (6); trial results will be available in 2010. Three other ARV-based microbicides, containing NNRTIs, are in earlier-phase trials (3, 5, 7-10). ARV-based microbicides are anticipated to be highly effective; however, there is concern that, if used by HIV-positive women, class-specific drug resistance may emerge. Here, we construct a mathematical model of a phase III clinical trial of an ARV-based microbicide. We use it to determine whether ARV-based microbicides that could potentially generate moderate to high levels of resistance (which we define as high-risk microbicides) could pass clinical testing. We then construct a mathematical model of heterosexual transmission and parameterize it using epidemiological, clinical, and behavioral data. "We use this model to predict the gender-specific epidemiological impact of wide-scale usage of high-risk and low-risk microbicides, where low-risk microbicides would only generate low levels of resistance.
Our distinction between high-risk and low-risk microbicides is in the probability that the ARVs contained in the microbicides will be systemically absorbed and subsequently have the potential to select for the emergence of drug-resistant strains of HIV." Currently, it is unknown whether ARV-based microbicides will be low-risk or high-risk. Increasing levels of systemic absorption of the ARV contained in the microbicide increase the possibility of drug resistance emerging. Assuming that ARV-based microbicides will be low-risk is reasonable because systemic absorption has not been found in phase I trials of intravaginal application of ARV-based microbicides (e.g., tenofovir, UC-781, and TMC-120) (3, 11, 12). However, assuming that they will be high-risk is also reasonable because the vagina is highly permeable (13-16) and is often an ideal route for drug administration (17-21). Hence, in all of our analyses, we investigate the potential impact of both types of microbicides.
We begin by constructing a mathematical model of a phase III efficacy trial of an ARV-based microbicide. Our model is constructed to match the original design of the dapivirine trial, which is a 12-month, two-arm, placebo-controlled, double-blind trial of about 10,000 women who are at high risk of HIV infection (e.g., female sex workers) with an average annual pretrial incidence of about 5%. The dapivirine trial protocol is once-daily application of microbicide regardless of the number of daily sex acts. This protocol will be used because it is expected that the concentration of dapivirine will be sustained over a 24-h period. Trial participants will be monitored for adherence throughout the trial. To conduct an ethical trial that will protect participants from acquiring drug resistance, they will be checked monthly for seroconversion using rapid testing with results available 10-15 min afterward. Seroconverters will be excluded from the trial to reduce their likelihood of developing drug resistance. However, the rapid tests are not highly sensitive to early detection because the test is based on HIV antibodies; therefore, there will be a 3- to 12-week window/lag period between when an individual becomes infected and when the test can detect their infection. Therefore, rapid testing will not detect early-stage seroconverters who will, because of their microbicide usage, be at risk of acquiring ARV resistance. Hence, even monthly testing may not prevent some seroconverters from acquiring drug resistance.
The timing of seroconversion will need to be accounted for when the trial results are analyzed.
Our clinical trial model enables us to track participants (separately in the placebo and microbicide arms) in four states: uninfected with HIV (state 1); recently infected with wild-type strains of HIV but with an undetectable infection (state 2); infected with detectable levels of wild-type strains of HIV (state 3); and infected with detectable levels of NNRTI-resistant virus (state 4). At the beginning of the trial all participants are in state 1. When the model is used to simulate a trial some participants in both arms of the trial (because the microbicide is not 100% effective) become infected and move from state 1 to state 2." Participants who are in the microbicide arm of the trial and in state 2 are not detected as seroconverters and are therefore at risk of acquiring resistance; hence, some HIV-infected participants who continue to use ARVbased microbicides acquire class-specific drug-resistant HIV and move directly to state 4." The remaining individuals in state 2 in the microbicide arm and all of the individuals in the placebo arm move to state 3 after 3-12 weeks. The model includes testing all participants for seroconversion and subsequent exclusion of seroconverters; seroconverters who are excluded are either in state 3 or state 4. It is assumed that before the trial participants either use condoms or no protection. We then allow participants in the microbicide arm of the trial to use one of four protection options: condoms, microbicides, condoms plus microbicides, or no protection (22). Each of the four options provides a different level of protection against infection; see SI Text for how these protection options are modeled. The model allows participants in the placebo arm to only use one of two protection options: condoms or no protection. Condom use during the trial is assumed not to change. The possibility of participants dropping out of the trial is also included. Further description of the model is given in Materials and Methods,
SI Text (section 2.1), and Fig. S1.
We used our clinical trial model to calculate the number of infections prevented and the number of participants who acquire drug resistance. We conducted two analyses, assuming that microbicides could be either low-risk or high-risk. For each of these analyses, we conducted 10,000 Monte Carlo simulations (23, 24) (see Materials and Methods and SI Text for further details). To conduct our simulations, we sampled ranges for all parameters rather than using point estimates; clinical data guided the estimation of any unknown parameters [see Table S2 and SI Text (section 2.2)]. This procedure allowed us to include estimation uncertainty and also use certain model parameters as experimental variables. The parameter that determined the risk level of the microbicides (i.e., the probability of systemic absorption) was varied from 0.01 to 0.03 for low-risk microbicides and from 0.5 to 0.9 for high-risk microbicides. Because the biological properties of ARV-based microbicides are unknown, we also varied two other parameters: (i) their clinical efficacy (i.e., the degree of protection that they will provide against HIV infection) (range 50-90%), and (ii) the length of time that it would take an HIV-infected women to acquire NNRTI resistance if the ARV is systemically absorbed (range 6 months to never). We also sampled adherence rates ranging from 0% to 100%, with 100% adherence signifying that microbicides are used daily [see SI Text (Table S2)]. To assess the potential impact of testing frequency on the number of cases of acquired resistance, we varied this parameter from weekly, fortnightly, monthly, every two months, every three months, every four months, every six months, to only once at the end of the trial (i.e., after 12 months).
"If microbicides are used in public health interventions and become widely available, they may be used by HIV-positive women who are unaware that they are infected. These HIVpositive women will be at risk of acquired resistance, and men will subsequently be at risk of transmitted resistance." We therefore constructed an epidemic model of heterosexual transmission and predicted, over a 10-year period, the potential genderspecific epidemiological impact of public health interventions based on using either low-risk or high-risk microbicides. Each analysis consisted of 10,000 Monte Carlo simulations (23, 24). Our model tracks men in three states: susceptible, infected with wild-type virus, or infected with NNRTI-resistant virus. It separately tracks two groups of women: women who use microbicides and women who choose not to use microbicides. Each group of women can also be in one of the three states: susceptible, infected with wild-type virus, or infected with NNRTIresistant virus. By tracking these nine states, we model both acquired and transmitted resistance. In our model, the women who are using microbicides and are infected with wild-type strains can develop acquired resistance (i.e., become infected with NNRTI-resistant strains). NNRTI-resistant strains can then be transmitted from women infected with NNRTI-resistant strains to uninfected men, and from men infected with NNRTIresistant strains to uninfected women. Our model structure also allows us to vary the coverage rate (i.e., the proportion of women using microbicides), adherence (for women using microbicides), and the relative fitness of NNRTI-resistant strains [see Materials and Methods, SI Text (section 3.1), Fig. S6, and Table S3 for a more detailed model description]. We used the model to predict the cumulative number of infections prevented, the incidence of transmitted resistance, the prevalence of resistance, and the cumulative number of infections prevented per resistant case. To make our predictions, we assumed that there would be no wide-scale, population-level surveillance and testing for seroconversion and resistance.
We parameterized our model using clinical, behavioral, and epidemiological data [see SI Text (section 3.2) and Table S3]. Microbicides were characterized by the same parameter ranges as in the clinical trial model, but in addition we included a fourth characteristic: clinical efficacy in protecting men against HIV infection. ARV-based microbicides are currently only being evaluated for efficacy in protecting women. However, it is biologically plausible that they may, by reducing viral loads in cervical-vaginal fluid in infected woman, protect men (to some degree) from infection (i.e., they may have clinical efficacy in protecting men who have sex with HIV-infected women). Clinical efficacy for each gender was treated as an experimental variable, varied between 0% and 90% and sampled independently [see SI Text (section 3.2) and Table S3].
"Results"
Our results show that the same number of infections will be prevented during a clinical trial regardless of whether the dapivirine-based microbicide (or any subsequently tested microbicide) is high-risk or low-risk (Table 1). Not surprisingly, the clinical efficacy of microbicides is the main determinant of the
number of infections prevented (see Figs. S3 and S4). However, the adherence rate is also extremely important in preventing infections; high rates will be extremely beneficial (Fig. 1A). Only 36 [median value; interquartile range (IQR) 11-100] infections will be prevented by the end of the dapivirine trial if adherence
is about 50% (median value; range 0-100%) (Table 1), but 133 (median value; IQR 96-171) infections will be prevented if adherence is 100%. We find that testing monthly for seroconversion (as planned for the dapivirine trial) will prevent the same number of infections as any other testing frequency.
Although high adherence will have a beneficial effect by preventing many infections (Fig. 1A), it could also have a very detrimental effect (if high-risk microbicides are evaluated) by generating a large number of resistant cases (Fig. 1B). The number of cases of acquired resistance that will develop during the dapivirine trial will mainly be determined by whether it (or any subsequently tested ARV-based microbicide) is high-risk or low-risk (Table 1 and Fig. S5), but high-risk microbicides will also be strongly affected by adherence (Fig. 1B). If adherence is high then a high-risk microbicide could generate many resistant cases
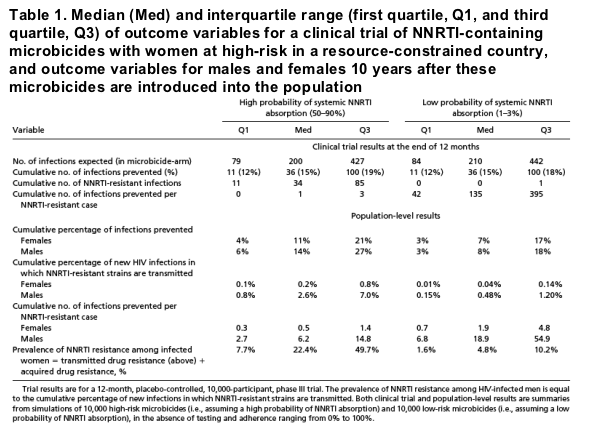
(Fig. 1B). However, if adherence is low then even a high-risk microbicide will only generate a few cases of resistance; for example, with about 14% adherence only about 3% of participants would acquire resistance, assuming that the average time for resistance to emerge is 18 months (Fig. 1B). If resistance emerges at a faster rate then the effect of adherence on high-risk microbicides will be even stronger (Fig. 1B). Low-risk microbicides will generate few resistant cases, even if adherence is high. For example, if adherence is 100% and the microbicide is low-risk, only about 0.6% of infections become drug-resistant (Table 1). For both high-risk and low-risk microbicides, increasing efficacy increases the benefit-to-risk profile in terms of the ratio of infections prevented per case of acquired resistance (Fig. S2). If the microbicide is low-risk the ratio will be very high (median 135; IQR 42-395), but if it is high-risk (and without testing) the ratio could be about 1 (median;IQR0-3) (Table 1).Wefound that varying dropout rates did not significantly affect any of our clinical trial results.
Monthly testing, to protect participants from the risk of acquiring resistance, is planned for the current phase III efficacy trials of dapivirine and other ARV-based microbicides. This protocol will require about 105,000 tests, including about 300 tests to detect resistance in seroconverters (Fig. 1C). Our results show that, with the planned protocol, almost no cases of resistance will arise if the microbicide is low-risk and only about 3 cases will arise if the microbicide is high-risk (Fig. 1C). Therefore, it will not be possible to determine whether dapivirine (or any other ARVbased microbicide that is tested by using this protocol) is high-risk or low-risk. Not surprisingly, less frequent testing would increase the number of resistant cases, but this effect will only be pronounced if a high-risk microbicide is being evaluated (Fig. 1C). For example, if there is no testing during the trial and the microbicide is high-risk, then 34 (median value) resistant cases could develop; but if the microbicide is low-risk, then only about 3 (median value) cases would arise (Table 1 and Fig. 1C, which assumes 100% adherence). These results show that frequent (i.e., monthly) testing is likely to mask the true risk of a high-risk microbicide to generate resistance. Consequently, high-risk microbicides could potentially pass through phase III clinical testing and subsequently be used in public health interventions.
If high-risk microbicides are used in public health interventions they could substantially increase levels of ARV resistance in a population (Table 1; predictions are from our epidemiological model simulations 10 years after an intervention has been initiated). Prevalence of resistance would be greatest in women (22% median; IQR 8-50%), but transmitted resistance would be about 12 times greater in men (2.6% median; IQR 0.8-7.0%) than in women (Table 1). However, if high-risk microbicides are even moderately efficacious (about 50%) they could be beneficial in reducing the HIV pandemic by decreasing transmission (Table 1). Surprisingly, our predictions show that both high-risk and low-risk microbicides could lead to greater reductions in incidence in men than in women (Table 1 and Fig. S7). In 74% of our simulations of high-risk microbicides and in 57% of our simulations of low-risk microbicides the cumulative number of infections prevented per resistant case was also greatest in men (Fig. S9). The black line in Fig. S7 shows the point at which the cumulative number of infections prevented is equally reduced for both men and women; the blue region to the right of the line indicates when microbicides would be more beneficial for men than women. These results show that, paradoxically, although vaginal microbicides are a female-initiated prevention tool designed to protect women against HIV infection, they could often prevent more infections in men. This phenomenon is more likely to occur if high-risk microbicides are used.
To determine which of the model's parameters were important in determining which gender would benefit the most from microbicides, we defined a male advantage threshold (MAT); at theMAT the cumulative number of infections prevented is equal in women and men. To identify the key parameters, we conducted a sensitivity analysis (23) on our simulated data and then fitted nonlinear response hypersurfaces (using multivariate regression) to our simulated epidemiological data [see SI Text (section 3.5) and Table S4]. Cross-products in the regression equations revealed interaction effects of the model's input parameters and caused curvature of the hypersurfaces (Fig. 2 and Fig. S8). We found that efficacy of the microbicide for women, efficacy of the microbicide for men, and the adherence rate were the three key parameters for both high-risk and low-risk microbicides. These parameters are important in determining the MAT because they strongly affect the number of infections prevented and the number of cases of acquired and transmitted resistance that develop. To elucidate where in the parameter space theMAT occurs, we examined each of the three possible pairing combinations of the key parameters. In Fig. 2, we present the results for when a high-risk microbicide is used in a public health intervention; the black line delimits the MAT and divides the parameter space into the regions where men will benefit the most and where women will benefit the most. Comparable results for a low-risk microbicide are shown in Fig. S8.
Interestingly, even if high-risk microbicides have no efficacy for men, men will still benefit more than women if the efficacy for women is less than about 50% (Fig. 2A). Men will also benefit the most if adherence is less than about 57% (Fig. 2B). Furthermore, even if women are 100% adherent, men will still benefit the most
if the efficacy for women is less than about 45% (Fig. 2C). Our results also show that men are more likely to have an advantage in terms of infections prevented if high-risk, rather than low-risk, microbicides are used (i.e., the MAT is shifted; compare Fig. 2 with Fig. S8). However, even if low-risk microbicides are used, the majority of the parameter region still favors men over women (Fig. S8). Taken together our results show that, for both high-risk and low-risk microbicides, men benefit more than women (i.e., a MAT exists) if microbicide efficacy in protecting women and/or adherence is low to moderate. Therefore, the conditions that lead to a MAT will not always occur but may be likely to arise. In terms of infections prevented per resistant case, both high-risk and low-risk microbicides will always benefit men more than women (Table 1 and Fig. S9). The degree of benefit for men decreases as the relative fitness of NNRTI-resistant strains increases (Fig. 2D).
Discussion
Safe, effective, and accessible HIV prevention methods are urgently needed. An HIV vaccine is not in the foreseeable future (25, 26), but ARV-based microbicides (which are currently in phase III clinical trials) could become widely available in 5-10 years (27). Our results indicate that adherence during efficacy trials of microbicides will greatly influence their efficacy results. The bias created by low adherence may in part explain the failure to show significant protective benefits of several prevention methods in efficacy trials (28). Because monthly testing will take place in the dapivirine trial, we predict that few, if any, cases of acquired resistance will arise during the trial even if the drug is readily absorbed (i.e., the microbicide is high-risk). Therefore, our analyses have shown that high-risk microbicides could pass phase III trials because their potential to cause resistance will be masked by frequent testing. We have also shown that it is possible that high levels of resistance could arise when high-risk microbicides are used as public health interventions and wide scale testing does not occur. Hence, monthly testing decreases a participant's risk of resistance during the trial but increases the risk of resistance for the general population when microbicides are used as interventions and very frequent testing does not occur. We recommend that it is essential that the degree of systemic absorption of the NNRTIs and the time for resistance to emerge should be measured in phase III efficacy trials. These factors should be measured in every trial because different NNRTIs are likely to be absorbed from microbicidal gels at different rates, and they should be well characterized before marketing authorization. It is also possible, but unlikely, that selection for resistance could occur in the vaginal cavity, and that systemic absorption would not be necessary. ARV-based microbicides, like any therapeutic product, may cause other adverse events apart from resistance that should also be monitored. They should also be evaluated during clinical trials to determine whether they show differential effectiveness against different HIV subtypes (29).
ARV-based microbicides protect against HIV infection by averting transmission in a similar manner as preexposure prophylaxis (PrEP). PrEP, based on a combination of tenofovir and another NRTI, emtricitabine, is currently being tested in clinical trials (30). It is currently unknown how effective PrEP will be,
and it could be possible that people will use PrEP and microbicides simultaneously to increase effectiveness. There are some similarities between ARV-based microbicides and PrEP. For individuals using either of these protection options, drugresistance may develop and so second- or third-line drug regimes could be required for therapy. Public health interventions based on PrEP could have similar population-level consequences for resistance as ARV-based microbicides and may also have some similar gender-specific differences. Important issues for both protection options include the responsibility to prevent secondary spread of drug-resistant infection and the need for frequent medical monitoring. However (unlike with condoms), it may well be possible to achieve high coverage and adherence to microbicides and/or PrEP because these interventions may be more acceptable to partners (27, 31). The cost-effectiveness of scaling up either intervention, which is currently unknown, will also be an important consideration in their public health assessment. The relative efficacies and expected adherence levels of both of these interventions will be crucial in evaluating their potential effectiveness and acceptability; these factors are still to be determined.
We have shown that even if ARV-based microbicides are high-risk and only moderately efficacious, they could reduce HIV incidence. Currently, only condoms and male circumcision are available for protection, and both are highly effective but not widely used. Therefore, the potential combined benefit of male circumcision, and/or condoms, together with the usage of microbicides could substantially reduce rates of heterosexual transmission. However, ARV-based microbicides could lead to relatively high levels of ARV resistance arising. Therefore, we recommend that careful and regular population-level monitoring occur to monitor for resistance if, and when, ARV-based microbicides are used in public health interventions. Our results imply that monitoring for resistance should occur when PrEP is widely used. However, it is important to note that if resistance does emerge as a result of either ARV-based microbicides or PrEP, the strains that will evolve will only be resistant to the specific class of drugs in the products (3, 32). Susceptibility to other classes of ARVs will be unaffected, and hence therapeutic options for the individuals who acquire resistance will be reduced but not eliminated. In addition, recently approved secondgeneration NNRTI drugs retain efficacy against most HIV variants that are resistant to first-generation NNRTI drugs (33, 34). Other ARV-based microbicides could use different classes of drugs such as entry inhibitors. CCR5 antagonists, such as the Rantes derivatives, have the potential to be highly cost-effective and less likely to select for resistance (35, 36).
Obviously, it is advantageous for microbicides to prevent infections in both men and women. However, vaginal microbicides are being developed for preventing infections in women and to empower women, particularly in resource-constrained
countries. They are a prevention tool that can only be used by women. Nevertheless, we have found that ARV-based microbicides may, under certain conditions, benefit men more than women. Whether this will occur will be determined by (i) the degree to which women adhere to microbicides, (ii) microbicide efficacy, and (iii) the viral fitness of drug-resistant strains. It is paradoxical that men may benefit more than women because of the development of effective microbicides; this paradoxical effect will be exacerbated if high-risk microbicides are used. We predict, therefore, that the population-level consequences of public health interventions that use ARV-based microbicides could well be surprising.
Materials and Methods
Weconstructed clinical trial and epidemic-level mathematical models. For the clinical trial model,wetracked 10,000women(female sex workers) at high risk of HIV infection in both a microbicide arm and a placebo-control arm; the pretrial average annual incidence was 5%. HIV transmission rates were calculated
according to per sex act transmission probabilities, the number of vaginal sex acts per partner, the average number of new sex partners per year, the protection afforded by condoms and microbicides, and the proportion of
acts in which each protection type is used. These factors were incorporated
into a mechanistic binomial formulation (37) to calculate the cumulative transmission probability per woman over the duration of the trial; see SI Text (section 2.1) for model equations and further description.
We also constructed an epidemiological model of heterosexual transmission in a high prevalence resource-constrained community where ARV-based microbicides are used in a public health intervention. The model is described
by a system of nine ordinary differential equations [see SI Text (section 3.1),
Fig. S6, and Table S3]. To identifymaximumbeneficial and detrimental effects, we modeled the introduction of microbicides into a treatment-naive population
without ambient resistance, and we did not include the possibility of large-scale testing for HIV infection.
|
|
| |
| |
|
|
|