| |
Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells
|
| |
| |
[BASIC SCIENCE: CONCISE COMMUNICATION]
AIDS:Volume 22(10)19 June 2008p 1131-1135
Archin, Nancy Ma; Eron, Joseph Ja; Palmer, Sarahb; Hartmann-Duff, Annea; Martinson, Jeffery Ac; Wiegand, Annb; Bandarenko, Nicholasa; Schmitz, John La; Bosch, Ronald Jd; Landay, Alan Lc; Coffin, John Mb; Margolis, David Ma
From the aUniversity of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA
bHIV Drug Resistance Program, NCI, NIH, Frederick, Maryland, USA
cRush-Presbyterian-St Luke's Medical Center, Chicago, Illinois, USA
dHarvard School of Public Health, Boston, Massachusetts, USA.
Abstract
Objectives: Valproic acid and intensified antiretroviral therapy may deplete resting CD4+ T-cell HIV infection. We tested the ability of valproic acid to deplete resting CD4+ T-cell infection in patients receiving standard antiretroviral therapy.
Methods: Resting CD4+ T-cell infection was measured in 11 stably aviremic volunteers twice prior to, and twice after Depakote ER 1000 mg was added to standard antiretroviral therapy. Resting CD4+ T-cell infection frequency was measured by outgrowth assay. Low-level viremia was quantitated by single copy plasma HIV RNA assay.
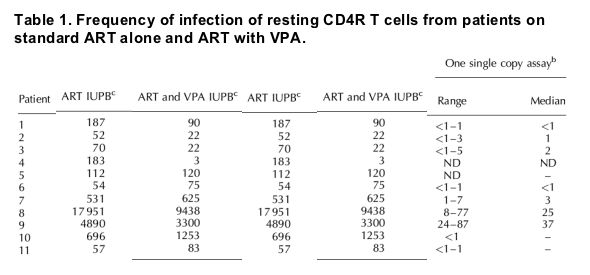
Results: A decrease in resting CD4+ T-cell infection was observed in only four of the 11 patients. Levels of immune activation and HIV-specific T-cell response were low and stable. Valproic acid levels ranged from 26 to 96 μg/ml when measured near trough. Single copy assay was performed in nine patients. In three patients with depletion of resting CD4+ T-cell infection following valproic acid, single copy assay ranged from less than 1-5 copies/ml. Continuous low-level viremia was observed in three patients with stable resting CD4+ T-cell infection (24-87, 8-87, and 1-7 copies/ml respectively) in whom multiple samples were analyzed.
Conclusion: The prospective addition of valproic acid to stable antiretroviral therapy reduced the frequency of resting CD4+ T-cell infection in a minority of volunteers. In patients in whom resting CD4+ T-cell infection depletion was observed, viremia was rarely detectable by single copy assay.
Introduction TOP
Despite its benefits, antiretroviral therapy (ART) cannot eliminate HIV infection [1-3]. Low levels of HIV RNA are detected in the plasma of many patients despite ART [4-6], and can be quantitated by a sensitive assay to limit of one copy of HIV RNA per milliliter [7]. Another barrier to the eradication of HIV infection is the early establishment of a latently infected pool of resting memory CD4+ cells [8-10].
Histone deacetylase (HDAC) is a critical regulator of HIV latency. The action of HDAC at the HIV promoter maintains quiescence of HIV gene expression in infected resting CD4+ lymphocytes [11-18]. HDAC inhibition leads to HIV outgrowth from resting CD4+ T cells of aviremic patients [19,20]. Resting CD4+ T-cell infection (RCI) was depleted when intensified ART was given in combination with valproic acid (VPA), an anticonvulsant and nonselective HDAC inhibitor [21]. We sought to test the ability of VPA to deplete RCI when combined with standard ART.
Methods
HIV-infected volunteers receiving stable ART with plasma HIV-1 RNA less than 50 copies/ml and a CD4 cell count of more than 300 cells/μl for at least 6 months were enrolled. Leukapheresis was performed on two occasions prior to the addition of VPA (on day -49 to -27 and day 0), and at 12 and 16 weeks after the addition of VPA (Depakote ER 1000 once a day). Other enrollment criteria have been described [21]; because of its interaction with VPA, volunteers receiving zidovudine were excluded. Informed consent was obtained from all patients.
Lymphocytes were obtained by continuous-flow leukapheresis. Isolation of resting CD4+ T cells, recovery and quantification of replication competent virus was performed as described [21]. Briefly, following purification resting CD4+ T cells were incubated for 2 days with the HIV integrase inhibitor L-870812 (1 _mol/l; gift of D. Hazuda, Merck Research Laboratory, West Point, Pennsylvania, USA) and efavirenz (15 nmol/l) or abacavir (4 _mol/l) as determined by treatment history to insure the decay of any preintegrated HIV DNA. A total of 93.6 million resting CD4+ T cells were plated in replicate dilutions of 2.5 million (36 cultures), 0.5 million (six cultures), and 0.1 million (six cultures) cells per well and stimulated with 1 μg/ml PHA-L (Remel, Lanexa, Kansas, USA), a five-fold excess of allogeneic irradiated peripheral blood mononuclear cells (PBMCs) from a seronegative donor, and 20 U/ml interleukin (IL-2) for 24 h. Cells were then washed and fed with CD8-depleted PBMCs collected from a selected HIV seronegative donor. These donor cells were screened for adequate CCR5 expression, collected by leukopheresis and stored in aliquots. Assays were performed with matched patient-donor pairs. Infected units per billion (IUPB) were estimated as described [21]. An observed depletion of greater than 50% is significant as this is greater than the 0.3 log10 standard deviation of our assay.
Flow cytometry analysis was performed on lymphocytes isolated at leukapheresis as described in refs [21-24]. Cell surface phenotypes were characterized as follows: naive T cells, CD45RA+ CD62L+; memory T cells, CD45RO+ CD45RA-; activated T cells, HLADR+ CD38+ or HLADR+ CD69+ CD25+. CD8+ and CD4+ T cells were analyzed.
Additional studies were performed in batch on leukocytes from patients 1 to 6 preserved by rate-controlled freezing, after thawing and overnight incubation. HIV-specific responses were analyzed as described in ref. [24] using individual 15-mer peptides from HIV-1 consensus subtype B Nef (49 peptides), HIV-1 consensus subtype B Gag (123 peptides), and HIV-1 consensus subtype B Pol (221 peptides) obtained from the National Institute of Health (NIH) AIDS Research and Reference Reagent program. Baseline T-cell activation was obtained on the media control samples. Cytokine production (IFN-_, IL-2) was also evaluated in the CD4+ and CD8+ T-cell populations.
Plasma HIV-1 RNA concentrations were measured by Roche Amplicor (Roche Molecular Systems, Branchburg, New Jersey, USA) with an assay detection limit of 50, and an ultra-sensitive, quantitative real-time, reverse transcriptase (RT)-PCR single copy assay (SCA) capable of detecting and quantifying plasma HIV-1 RNA to a limit of detection of 1 copy/ml [25]. Seminal HIV-1 RNA was measured by NucliSens QT (bioMerieux, Durham, North Carolina, USA) with an assay detection limit of 400 copies/ml. Seminal fluid was collected at the same time points as leukapheresis. SCA was performed on serum samples collected at the screening visit, at 27-49 days before the start of VPA therapy, on days 0, 3, and weeks 1, 2, 4, 8, 12, and 16 after the start of VPA therapy.
Results
Twelve HIV-infected men, stably suppressed on ART, entered this study. ART included protease inhibitors (five patients), nonnucleoside reverse transcriptase inhibitors (six patients), or both (one patient). CD4 cell counts at entry ranged from 581 to 1121 cells/μl (27-52%).
After determination of the frequency of resting cell infection circulating at two time points, 1000 mg VPA (Depakote ER) was administered once a day for 16 weeks. One patient was removed from the study prior to the evaluation of VPA effect for protocol violations.
Total VPA levels were measured near trough, prior to daily dosing, and ranged from 26 to 96 μg/ml. Dose was increased if trough levels were consistently below 40 μg/ml; adjustment to 1250 mg was required in one patient. VPA therapy was generally well tolerated. Four patients reported minor adverse effects that resolved without specific intervention. Patient 8 reported depressive symptoms not clearly related to study drug.
CD4+ and CD8+ cells showed no changes in CD38+ HLADR+ (data not shown). In the first six patients, studies of HIV-specific immune responses were undertaken. VPA did not alter the proportions of naive, effector, or central memory CD4+ or CD8+ T cells (data not shown). HIV-specific T-cell responses to viral peptide antigen were low and unaltered following VPA therapy (data not shown).
VPA therapy combined with standard ART depleted resting cell infection in only four of the 11 patients studied (Table 1). Median VPA level at trough was not significantly different in patients with RCI depletion (56 μg/ml) compared with those without (49 μg/ml).
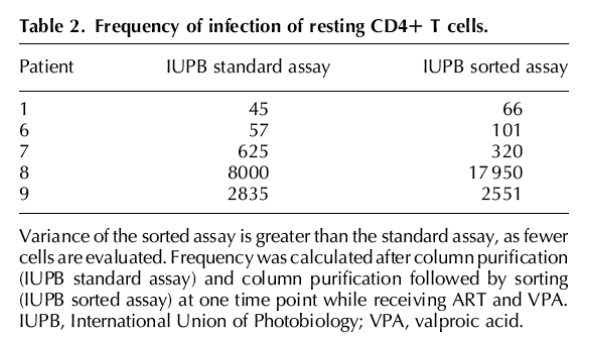
Given the observation of intermittent or low-level viremia in some patients, additional assays were performed to insure that outgrowth represented integrated provirus. After negative selection over antibody columns, when sufficient cells were available additional cell sorting was performed to obtain resting CD4+ populations of more than 99.9% purity in five patients. As fewer cells (34-49 million resting CD4+ T cells) were available after sorting, the point estimate of IUPB obtained in this assay is less precise. However, the frequency of RCI in these highly purified cell preparations did not differ significantly from a parallel assay performed without additional sorting (Table 2) or from the pooled results of the standard assay (Table 1).
To measure more accurately the extent of viremia to a limit of detection of 1 copy/ml, SCA was performed. SCA measurements correlated with Amplicor results (Table 1). In patient 9, intermittent viremia of more than 50 copies/ml by Amplicor assay was observed. In this patient SCA was routinely positive at 24-87 copies/ml. Therefore in this patient, results appeared consistent with variation in PCR-based detection of stable low-level viremia, as described in a longitudinal study [26]. In contrast, in patient 8 lack of viremia detected by Amplicor assay early in the study was associated with viremia of less than 30 copies/ml by SCA, whereas after week 8 clinical suspicions of nonadherence and intermittent Amplicor assays of more than 50 copies/ml were associated with SCA of at least 68 copies/ml.
We did not observe an association between duration of aviremia and RCI depletion. Four patients with depletion of RCI had HIV RNA less than 50 copies/ml for a mean of 15 months (median 13, range 10-23), compared with a mean of 19 months (median 14, range 7-45) in seven patients without depletion of RCI.
An association was observed between residual or low-level viremia and the stable RCI following VPA therapy. Depletion was not observed in patients 8 and 9, in whom intermittent viremia was observed, nor in patient 7 who had residual viremia by SCA (Table 1). Residual viremia was rarely detectable in patients 6, 11, and 12, although only in patient 6 were we able to perform the SCA repeatedly. In contrast, viral load was repeatedly less than 5 copies/ml in all three patients with a significant decline in RCI in whom the SCA was performed (patients 1, 2, and 3).
In some patients, viremia may be detected in genital fluids when it is not detectable in plasma [27]. Seminal fluid was provided by patients 1, 3, 5, 6, and 8, but HIV RNA was undetectable (limit of detection 400 copies/ml) at all available time points.
Discussion
Current antiretroviral therapy does not eliminate persistent resting cell infection. Therapies that selectively target the quiescent provirus and induce its expression could open new avenues for combating persistent HIV infection.
We studied the effect of the addition of VPA to standard ART on RCI. There was a decline in RCI of more than 50% in four of 11 patients receiving VPA therapy combined with standard ART. We did not observe large fluctuations or an apparent increase in resting CD4+ T-cell infection after the addition of VPA to ART, as reported [28].
Our observations suggest that in the majority of patients on standard ART, VPA alone is not sufficient to reduce RCI. Notably, there appeared to be an association of a decline in RCI with the absence of low-level viremia. Jones and Perelson [29] have recently suggested that intermittent low-level viremia to levels above approximately 7 copies/ml may prevent the decay of chronically infected cell populations despite continuous ART. Plasma viremia was observed to be uniformly less than 5 copies/ml in all three patients with a significant decline of RCI in whom SCA was performed. However, SCA was less than 5 copies/ml in three patients without a decline in RCI, although a limited number of assays were available in these patients.
One potential result of the induction of expression of latent provirus might be the appearance of low-level viremia in the peripheral blood, if viral expression was robust enough to escape the tissue reservoir and appear in the peripheral circulation. We did not observe evidence of a change in low-level viremia as measured by SCA throughout VPA therapy, similar to the stability of low-level viremia in patients treated with ART, VPA, and enfuvirtide [21].
Cells with an activated phenotype or HIV-specific immune cells might appear in the peripheral blood following induction of latent provirus. However, we found the number of these cells to be low, as is common in patients stably suppressed on ART, and without correlation to the level of RCI after VPA therapy.
Further depletion of RCI by VPA or response to VPA in a larger subset of patients might be observed if higher doses of VPA were administered. However, these modest findings suggest that alternate approaches may be needed to substantially deplete RCI in the majority of HIV-infected patients with stable suppression of viremia. If therapies that induce remission or eradication of HIV infection are ever achieved, they will have to be effective over periods of treatment measured in months rather than years. Alternate approaches to intensify ART or utilize more potent HDAC inhibitors [30,31] are in development.
|
|
| |
| |
|
|
|