| |
Subgroup and Resistance Analyses of Raltegravir for Resistant HIV-1 Infection
|
| |
| |
NEJM July 24, 2008
David A. Cooper, M.D., D.Sc., Roy T. Steigbigel, M.D., Jose M. Gatell, M.D., Ph.D., Jurgen K. Rockstroh, M.D., Christine Katlama, M.D., Patrick Yeni, M.D., Adriano Lazzarin, M.D., Bonaventura Clotet, M.D., Princy N. Kumar, M.D., Joseph E. Eron, M.D., Mauro Schechter, M.D., Ph.D., Martin Markowitz, M.D., Mona R. Loutfy, M.D., M.P.H., Jeffrey L. Lennox, M.D., Jing Zhao, Ph.D., Joshua Chen, Ph.D., Desmond M. Ryan, B.S., Rand R. Rhodes, M.S., John A. Killar, M.S., Lucinda R. Gilde, B.S., Kim M. Strohmaier, B.S., Anne R. Meibohm, Ph.D., Michael D. Miller, Ph.D., Daria J. Hazuda, Ph.D., Michael L. Nessly, M.S., Mark J. DiNubile, M.D., Robin D. Isaacs, M.D., Hedy Teppler, M.D., Bach-Yen Nguyen, M.D., for the BENCHMRK Study Teams
ABSTRACT
Background We evaluated the efficacy of raltegravir and the development of viral resistance in two identical trials involving patients who were infected with human immunodeficiency virus type 1 (HIV-1) with triple-class drug resistance and in whom antiretroviral therapy had failed.
Methods We conducted subgroup analyses of the data from week 48 in both studies according to baseline prognostic factors. Genotyping of the integrase gene was performed in raltegravir recipients who had virologic failure.
Results Virologic responses to raltegravir were consistently superior to responses to placebo, regardless of the baseline values of HIV-1 RNA level; CD4 cell count; genotypic or phenotypic sensitivity score; use or nonuse of darunavir, enfuvirtide, or both in optimized background therapy; or demographic characteristics. Among patients in the two studies combined who were using both enfuvirtide and darunavir for the first time, HIV-1 RNA levels of less than 50 copies per milliliter were achieved in 89% of raltegravir recipients and 68% of placebo recipients. HIV-1 RNA levels of less than 50 copies per milliliter were achieved in 69% and 80% of the raltegravir recipients and in 47% and 57% of the placebo recipients using either darunavir or enfuvirtide for the first time, respectively. At 48 weeks, 105 of the 462 raltegravir recipients (23%) had virologic failure. Genotyping was performed in 94 raltegravir recipients with virologic failure. Integrase mutations known to be associated with phenotypic resistance to raltegravir arose during treatment in 64 patients (68%). Forty-eight of these 64 patients (75%) had two or more resistance-associated mutations.
Conclusions When combined with an optimized background regimen in both studies, a consistently favorable treatment effect of raltegravir over placebo was shown in clinically relevant subgroups of patients, including those with baseline characteristics that typically predict a poor response to antiretroviral therapy: a high HIV-1 RNA level, low CD4 cell count, and low genotypic or phenotypic sensitivity score. (ClinicalTrials.gov numbers, NCT00293267 [ClinicalTrials.gov] and NCT00293254 [ClinicalTrials.gov])
Despite the substantial decrease in mortality and morbidity rates associated with highly active antiretroviral therapy over the past decade, there is still a substantial need for effective antiretroviral drugs for patients infected with resistant human immunodeficiency virus type 1 (HIV-1).1,2 The majority of licensed antiretroviral drugs belong to three classes targeting either the HIV-1 protease or reverse transcriptase, and considerable cross-resistance exists among drugs within each class.3,4 In patients with resistant virus, use of antiretroviral agents from new classes offers considerable potential benefit because of the absence of cross-resistance.5,6,7
HIV-1 integrase represents a new therapeutic target.8,9 Consequently, HIV-1 integrase inhibitors would be expected to retain activity against HIV-1 that is resistant to other classes of antiretroviral drugs. Raltegravir (MK-0518; Isentress, Merck), an HIV integrase strand-transfer inhibitor, has exhibited substantial efficacy and a favorable safety profile in patients infected with HIV-1,7,10,11 including those with multidrug-resistant HIV-1 and a history of treatment failure.7,11 In a phase 2 study of previously treated patients who had multidrug-resistant virus, mean decreases in the viral load at week 24 ranged from 1.8 to 1.9 log10 copies per milliliter in those receiving raltegravir at doses of 200, 400, or 600 mg twice daily in combination with optimized background therapy, as compared with 0.4 log10 copies per milliliter in those receiving optimized background therapy alone; in approximately 60% of patients receiving raltegravir, as compared with 13% of patients receiving placebo, HIV-1 RNA levels were reduced to less than 50 copies per milliliter.7 These findings were confirmed in both phase 3 BENCHMRK-1 and BENCHMRK-2 studies (Blocking Integrase in Treatment Experienced Patients with a Novel Compound against HIV, Merck studies).11 In this report, we present the results of subgroup efficacy analyses and the evaluation of virologic resistance to raltegravir in patients with virologic failure, using data from week 48 from the combined BENCHMRK-1 and BENCHMRK-2 studies.
Methods
The studies were designed, managed, and analyzed by the sponsor in conjunction with the academic authors. The authors had access to all study data on request. This report was principally drafted by two academic authors and four industry authors and was critically reviewed and approved by all the authors in its final form before submission. All authors vouch for the completeness and accuracy of the data.
Study Design
The identical study design of BENCHMRK-1 and BENCHMRK-2, criteria for scoring the activity of optimized background therapy, and the overall evaluation of efficacy are described in detail in the article by Steigbigel et al. in this issue of the Journal.11 The subgroup efficacy analyses included complete 48-week data from both studies. The potential emergence of resistance to raltegravir was investigated in patients with virologic failure by genotyping the integrase coding sequence. The integrase gene was reverse-transcribed from plasma HIV-1 RNA and sequenced according to standard methods. Consensus amino acid sequences were compared with pretreatment genotypes.
Statistical Analysis
Because BENCHMRK-1 and BENCHMRK-2 shared the same study design and showed consistent treatment effects, a combined analysis and exploratory subgroup analyses of pooled data from the two studies were undertaken. Subgroup analyses based on potential prognostic factors were prespecified (except for the combination of enfuvirtide and darunavir in optimized background therapy) before unblinding of the data. The phenotypic and genotypic sensitivity scores are the total number of antiretroviral drugs used as part of the optimized background therapy to which a patient's HIV was fully susceptible, as determined with the use of phenotypic and genotypic resistance testing, respectively. Enfuvirtide use in optimized background therapy in patients who had not previously received enfuvirtide was counted as one active drug in optimized background therapy and added to the genotypic and phenotypic sensitivity scores. Darunavir use in optimized background therapy in patients who had not previously received darunavir was likewise counted as one active drug and added to the phenotypic and genotypic sensitivity scores. In addition, darunavir use in optimized background therapy in patients who had not previously received darunavir was counted as one active protease inhibitor.
Prognostic factors, such as baseline HIV-1 RNA levels and the genotypic and phenotypic sensitivity scores for optimized background therapy, may be strongly associated with virologic and immunologic responses but are less likely to be associated with adverse events or discontinuation for reasons not related to treatment. Because it predominantly reflects the antiretroviral effect of treatment, an observed-failure approach (in which only patients who discontinued the study because of lack of efficacy were considered to have treatment failure at subsequent time points) was used as the basis of the exploratory subgroup analyses.7 Differences (and the 95% confidence intervals) between treatment groups in the proportion of patients with HIV-1 RNA levels below 50 copies per milliliter and in the mean change from the baseline CD4 cell counts were calculated for both the overall study groups and subgroups of interest.11
Results
Characteristics of the Patients
A total of 699 patients were treated in BENCHMRK-1 and BENCHMRK-2: 462 patients received raltegravir and 237 received placebo. Baseline characteristics were generally balanced between the treatment groups within each study.11 Table 1 lists selected baseline characteristics that have been identified as factors potentially affecting the efficacy of antiretroviral therapy. These covariates include the baseline HIV-1 RNA level and CD4 cell count; the use or nonuse of newly available antiretroviral drugs such as enfuvirtide, darunavir, and tipranavir as part of optimized background therapy; the number of active protease inhibitors used in optimized background therapy; and the number of active drugs in optimized background therapy, as measured by the phenotypic or genotypic sensitivity score at baseline.
Table 1. Selected Baseline Characteristics of the Patients in BENCHMRK-1 and BENCHMRK-2.
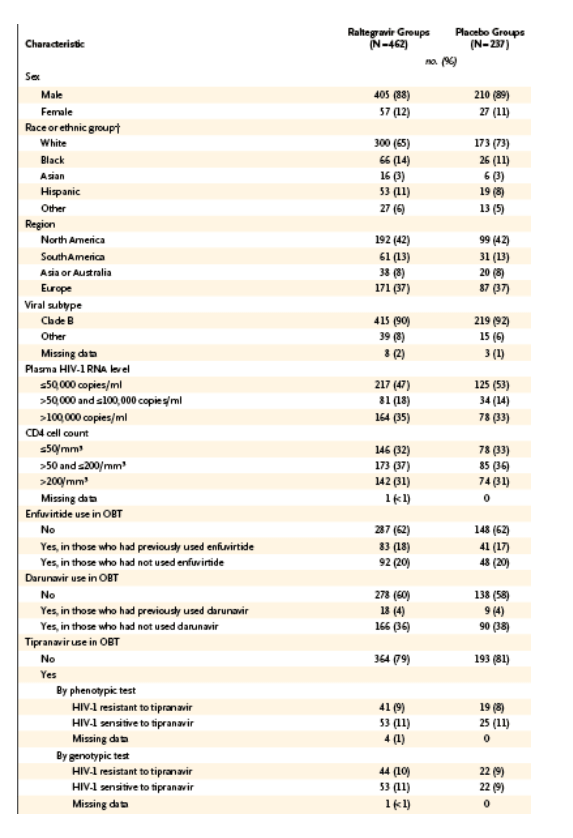
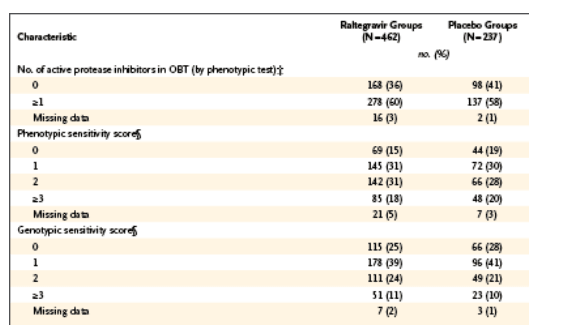
Baseline HIV-1 RNA levels were greater than 100,000 copies per milliliter in 35% of raltegravir recipients and 33% of placebo recipients. Baseline CD4 cell counts were 50 or less per cubic millimeter in 32% and 33% of raltegravir and placebo recipients, respectively. The median number of antiretroviral drugs in the optimized background therapy was four in both treatment groups. Despite this aggressive treatment, 36% of patients in the raltegravir group and 41% of patients in the placebo group had no fully active protease inhibitors in their optimized background therapy. The genotypic sensitivity score of the optimized background therapy was 0 in 25% of patients receiving raltegravir and in 27% of patients receiving placebo. The phenotypic sensitivity score of the optimized background therapy was 0 in 15% of raltegravir recipients and in 19% of placebo recipients.
Subgroup Efficacy Analyses
As reported in the article by Steigbigel et al.,11 the overall virologic and immunologic responses in the raltegravir groups were superior to those in the placebo groups after 16 weeks and 48 weeks of treatment. At week 48, in the analysis in which noncompletion constituted treatment failure, 72% of patients in the raltegravir groups, as compared with 37% of patients in the placebo groups, had HIV-1 RNA levels below 400 copies per milliliter (P<0.001), and 62% of patients in the raltegravir groups, as compared with 33% of patients in the placebo groups, had HIV-1 RNA levels below 50 copies per milliliter (P<0.001). Similar results were seen in the analyses that used the observed-failure approach.
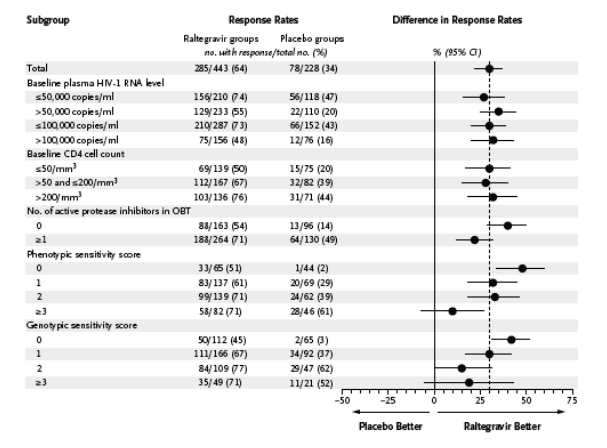
In subgroup analyses involving selected baseline prognostic factors and demographic characteristics based on the observed-failure approach, raltegravir recipients had higher virologic and immunologic response rates than placebo recipients across most subgroups, including patients with baseline HIV-1 RNA levels of more than 100,000 copies per milliliter and CD4 cell counts of less than 50 per cubic millimeter (Figure 1). In patients receiving optimized background therapy with a genotypic sensitivity score of 0, HIV-1 RNA levels of less than 50 copies per milliliter were achieved at week 48 in 45% of patients receiving raltegravir as compared with 3% of those receiving placebo, and the mean changes in the CD4 cell count between baseline and week 48 were 81 and 11 per cubic millimeter, respectively (Figure 2). Among patients with more active optimized background therapy, reflected by a genotypic sensitivity score of 2, 77% of raltegravir recipients had HIV-1 RNA levels below 50 copies per milliliter at week 48, as compared with 62% of placebo recipients; the corresponding mean changes in the CD4 cell count were 145 and 87 per cubic millimeter, respectively. Similar findings were observed in the subgroup analysis based on the phenotypic sensitivity score. Only in the relatively few patients whose optimized background therapy was associated with a genotypic or phenotypic sensitivity score of 3 or more was the treatment advantage of raltegravir over placebo not strikingly evident.
Figure 1. Percentages of Patients with HIV-1 RNA Levels of Less Than 50 Copies per Milliliter at Week 48, According to Subgroup.
The vertical dashed line represents the overall treatment-effect estimate. OBT denotes optimized background therapy.
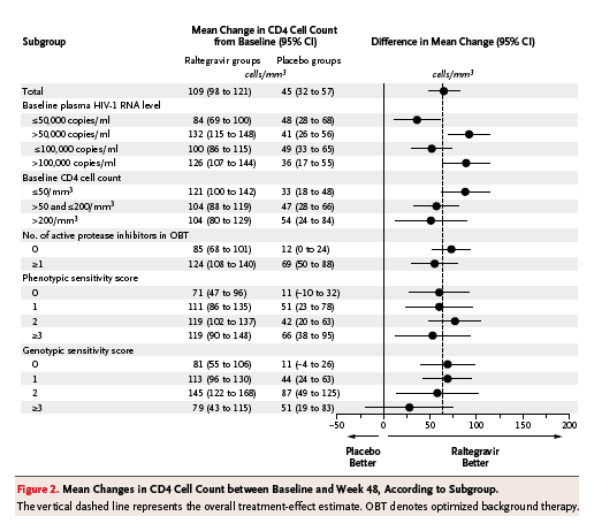
Additional analyses were conducted to evaluate the effect of newly available antiretroviral drugs (darunavir or enfuvirtide used alone or in combination, and tipranavir) as part of optimized background therapy. Among patients receiving both enfuvirtide and darunavir for the first time, 89% of patients in the raltegravir groups and 68% of patients in the placebo groups had HIV-1 RNA levels of less than 50 copies per milliliter at week 48 (Figure 3), with corresponding changes in the CD4 cell count of 129 and 81 per cubic millimeter, respectively (Figure 1 in Supplementary Appendix 2, available with the full text of this article at www.nejm.org). HIV-1 RNA levels below 50 copies per milliliter were achieved in 69% and 80% of the raltegravir recipients as compared with 47% and 57% of the placebo recipients using either darunavir or enfuvirtide for the first time, respectively, and the changes in CD4 cell count were 114 and 116 per cubic millimeter among raltegravir recipients as compared with 74 and 49 per cubic millimeter among placebo recipients, respectively.
Figure 3. Percentages of Patients with HIV-1 RNA Levels of Less Than 50 Copies per Milliliter at Week 48, According to Use or Nonuse of Selected Antiretroviral Drugs.
The vertical dashed line represents the overall treatment-effect estimate. Data from patients treated with enfuvirtide or darunavir as part of their optimized background therapy were excluded from this analysis if they had previously been treated with enfuvirtide or darunavir, respectively. OBT denotes optimized background therapy.
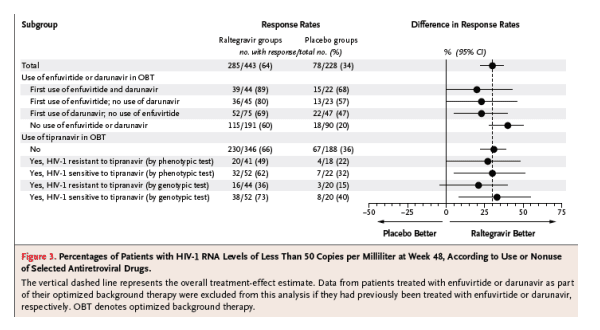
Raltegravir recipients had higher response rates than placebo recipients, whether or not tipranavir was used in the optimized background therapy.12 In patients treated with tipranavir who had virus that was genotypically sensitive to tipranavir, HIV-1 RNA levels were reduced to less than 50 copies per milliliter in 73% of raltegravir recipients, as compared with 40% of placebo recipients (Figure 3). The mean changes in the CD4 cell count in this subgroup were 114 and 56 per cubic millimeter, respectively (Figure 1 in Supplementary Appendix 2). In patients treated with tipranavir as part of their optimized background therapy who had virus that was genotypically resistant to tipranavir, 36% of raltegravir recipients, as compared with 15% of placebo recipients, had HIV-1 RNA levels of less than 50 copies per milliliter; the corresponding mean changes in the CD4 count were 79 and 12 per cubic millimeter, respectively.
Additional efficacy analyses based on sex, race or ethnic group, geographic region, and viral subtype consistently showed higher response rates in the raltegravir groups than in the placebo groups, in terms of viral-load reductions (Figure 4) as well as increases in the CD4 cell count (Figure 2 in Supplementary Appendix 2).
Resistance Mutations in the Integrase Gene
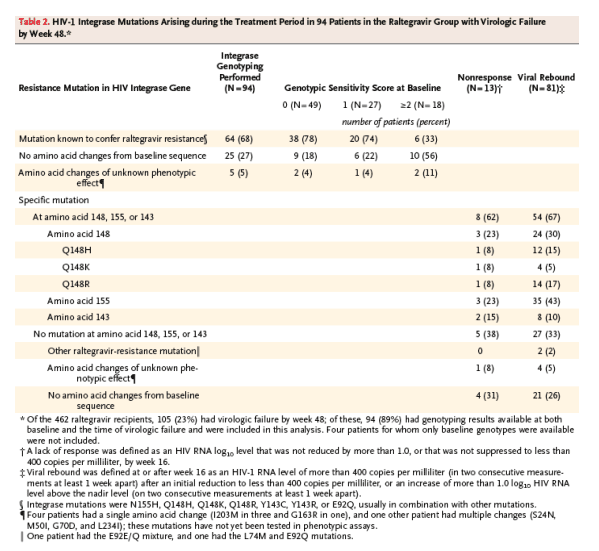
A total of 105 of the 462 patients receiving raltegravir (23%) had virologic failure by week 48. Integrase genotyping had been performed both at baseline and after virologic failure in 94 of these 105 patients (90%) at the time of this analysis; 64 of the 94 patients (68%) had genotypic evidence of viral resistance to raltegravir when tested at the time of, or shortly after, viral rebound (Table 2). Of the 64 patients with integrase mutations previously associated with phenotypic raltegravir resistance, 48 (75%) had two or more such mutations. Virologic failure was generally associated with mutations at one of three residues - Y143, Q148, or N155 - usually in combination with at least one other mutation (Table 2). The risk of mutations arising during treatment was increased in patients with higher baseline HIV-1 RNA levels or receiving optimized background therapy with a genotypic or phenotypic sensitivity score of 0.
Discussion
The results of the BENCHMRK-1 and BENCHMRK-2 studies showed the consistently superior efficacy of raltegravir over placebo when used in combination with optimized background therapy.11 Substantial viral-load suppression and increases in CD4 cell counts as compared with the baseline values were sustained for at least 48 weeks. The absolute response rate in the combined placebo groups (33% of patients having <50 HIV-1 RNA copies per milliliter) was better than that in a phase 2 study of raltegravir (13% with <50 HIV-1 RNA copies per milliliter),7 most likely reflecting the permitted use of investigational or recently licensed antiretroviral drugs in optimized background therapy in the BENCHMRK studies. The response rate in the placebo groups in these studies was consistent with the 45% response rate reported previously among similar patients receiving darunavir.13
The BENCHMRK studies were not powered to show significant effects within subgroups.14 Although there may have been imbalances within each subgroup in other prognostic factors between the raltegravir groups and the placebo groups, the subgroup analyses consistently showed a treatment advantage of raltegravir over placebo when given in combination with optimized background therapy. Specifically, raltegravir was more efficacious than placebo, regardless of baseline prognostic factors. Overall, patients with lower baseline HIV-1 RNA levels or higher baseline CD4 cell counts had higher response rates than patients with higher baseline HIV-1 RNA levels or lower baseline CD4 cell counts; however, the differences in treatment effect between the raltegravir and placebo groups were consistent in direction and magnitude in these subgroups. These findings are similar to those from the T-20 vs. Optimized Regimen Only Studies (TORO-1 and TORO-2) of enfuvirtide.5,6,15
As found in the phase 2 trial of raltegravir in previously treated patients infected with a multidrug-resistant virus,7 raltegravir had superior efficacy as compared with placebo in patients receiving optimized background therapy with a genotypic or phenotypic sensitivity score of 0, which is generally regarded as the most challenging treatment scenario. Nonetheless, functional monotherapy with raltegravir (in which there are no fully active drugs in the optimized background therapy) should be avoided whenever possible, given the greater absolute response rates among patients with more active optimized background therapy (defined as genotypic or phenotypic sensitivity score >0) as well as the lower risk of virologic failure and development of resistance in patients receiving more active optimized background therapy. For example, in 89% of patients receiving raltegravir along with enfuvirtide and darunavir for the first time in their optimized background therapy, HIV-1 RNA levels were reduced to less than 50 copies per milliliter. This substantial rate of viral suppression, which approaches the rates reported among patients infected with HIV who have not been previously treated,16 is remarkable for patients infected with virus that has triple-class drug resistance and in whom therapy has failed previously. The BENCHMRK studies, along with other recently reported studies,13,17 indicate that suppressing the viral load to less than 50 copies per milliliter is an attainable goal, even in heavily pretreated patients infected with virus that has multiclass drug resistance.
In healthy volunteers, coadministration of raltegravir with tipranavir reduced nadir levels of raltegravir by 55%.12 Therefore, a detailed analysis of tipranavir use in the phase 3 BENCHMRK studies was undertaken to evaluate the potential effect on the efficacy of raltegravir. Among patients who received tipranavir as part of optimized background therapy in both the raltegravir and the placebo groups, those with tipranavir-resistant virus had lower response rates than those with tipranavir-sensitive virus, presumably owing to the lack of an active protease inhibitor in the tipranavir-based optimized background therapy. In patients with tipranavir-sensitive virus, the use of tipranavir as part of optimized background therapy did not appear to negate the favorable treatment effect in the raltegravir groups.
Sex, race or ethnic group, geographic region, and viral subtype (clade B vs. other clades) apparently did not affect overall efficacy. Subgroup analyses according to sex, race or ethnic group, geographic region, and viral subtype showed generally higher response rates for raltegravir recipients than for placebo recipients, but more research is necessary to confirm these observations.
The limited genotypic-resistance data available to date support the in vitro findings that more than one integrase mutation is generally observed in HIV strains that become resistant to raltegravir.9 Genotypic resistance to raltegravir was anticipated in patients who had virologic failure in the raltegravir groups, especially in those who received raltegravir as functional monotherapy. Consequently, as recommended in formal treatment guidelines for all antiretroviral drugs,1,2 raltegravir should be used in combination with at least one other active antiretroviral agent whenever possible to minimize the development of resistance and the risk of virologic failure. Long-term efficacy data, beyond 48 weeks, particularly in patients receiving optimized background therapy with genotypic and phenotypic sensitivity scores of 0, will aid in the interpretation of specific integrase mutations and their contribution to the development of resistance to raltegravir.
In summary, treatment with raltegravir in combination with optimized background therapy, as compared with optimized background therapy alone, resulted in significant virologic and immunologic benefits for at least 48 weeks in both international BENCHMRK studies. The subgroup analyses presented here show a consistently favorable treatment effect of raltegravir, regardless of viral load, CD4 cell count, and genotypic or phenotypic sensitivity score at baseline and regardless of whether enfuvirtide, darunavir, or both were included in the optimized background therapy. Our data provide evidence that raltegravir will be a valuable addition to the current armamentarium for the treatment of patients infected with multidrug-resistant HIV-1.
Supported by Merck.
|
|
| |
| |
|
|
|