| |
Hepatitis C Virus Coinfection Does Not Affect
CD4 Restoration in HIV-Infected Patients
after Initiation of Antiretroviral Therapy
|
| |
| |
AIDS RESEARCH AND HUMAN RETROVIRUSES July 2008
Kari Yacisin, Wake Forest University Health Sciences, Winston Salem, North Carolina 27157.
Ivana Maida, Hospital Carlos III, Madrid, Spain. Istituto Maladie Infettive, University of Sassari, Sassari, Italy.
Maria Jose Rios, Hospital Virgen de la Macarena, Seville, Spain.
Vincent Soriano, Hospital Carlos III, Madrid, Spain.
Marina Nunez, Wake Forest University Health Sciences, Winston Salem, North Carolina 27157.
Abstract
There are conflicting data regarding the influence of hepatitis C virus (HCV) infection on the immune restoration experienced by HIV-infected patients who receive highly active antiretroviral therapy (HAART). In this multicenter, retrospective, longitudinal study, CD4 restoration was assessed according to HCV status in treatment- naive HIV-infected patients within 3 years of HAART. Only patients with persistent HIV suppression were included. Factors predicting CD4 gains were analyzed with multivariate linear regression. Out of 322 patients included 139 had positive HCV-RNA and 183 were only HIV infected. HCV-HIV-coinfected patients were older, more often ex-intravenous drug users (IVDU), and had less advanced HIV infection.
Baseline CD4 count [OR -0.21 [95% CI (-0.34)-(-0.04)]; p=0.01] and male sex [OR -0.19 [95% CI (-191.12)-(-10.87)]; p = 0.03] predicted smaller increments in absolute CD4 counts, and higher baseline CD4% [OR -0.38 [95% CI (-0.39)-(-0.21)]; p <0.0001] and older age [OR -0.12 [95% CI (-0.23)-(-0.01)]; p=0.03] predicted smaller gains in CD4% after 3 years of HAART.
A history of IVDU was associated with smaller absolute CD4 count increases at 1 year of therapy [OR -0.20 [95% CI (-128.32)-(-16.24)]; p=0.01]. Use of nucleoside reverse transcriptase inhibitor (NRTI)-only regimens and of zidovudine as part of the NRTI backbone was associated with smaller and greater gains in CD4%, respectively. HCV replication per se does not impair the CD4 restoration in HIV-infected patients successfully treated with antiretroviral therapy. Lower baseline CD4 counts are the strongest predictors of greater CD4 gains over a 3-year period, while a history of IVDU negatively affects CD4 restoration only early after the initiation of HAART.
Introduction
THERE ARE CONFLICTING DATA regarding the influence of HCV coinfection on CD4 restoration seen in HIV+ subjects treated with highly active antiretroviral therapy (HAART). While some studies suggest that HIV/HCV-coinfected
patients might have an impaired immune response under HAART compared to HIV-monoinfected subjects,1-6 other studies do not support those findings.7-11
A limitation in most of the studies is that HCV antibodies (HCV-Ab) rather than plasma HCV-RNA levels were used to define the HCV status. While the relationship between HCV viremia and CD4 restoration has been assessed in a few studies, patients with negative HCV RNA were not included for analysis.5,9,12,13 On the other hand, other common factors among HCV-Ab-positive HIV-infected patients, such as a history of intravenous drug use (IVDU), might have acted as confounders.14-17 The purpose of this study was to evaluate the impact of active HCV replication, and of other factors, on CD4 restoration in treatment-naive HIV-infected individuals successfully treated with HAART over a 3-year period.
Materials and Methods
This is a retrospective, cohorts study performed at two institutions (Wake Forest University Baptist Medical Center, Winston-Salem, NC and Hospital Carlos III, Madrid, Spain). Approval from the institutional review board at each institution was obtained. Data were recorded between 2005 and 2007.
Treatment-naive HIV-infected patients who initiated HAART between 2000 and 2005 and who achieved unde-tectable HIV RNA levels were identified at the two institutions from two existing databases. Patients were eligible if they had been regularly followed at the corresponding HIV clinic, had maintained undetectable HIV-RNA levels (sporadic blips with viral load below 500 copies/ml were accepted) after initiation of HAART for at least 2 years, and had been tested for anti-HCV (HCV-Ab) and also for HCV RNA if HCV-Ab was positive. Exclusion criteria included concurrent anti-HCV therapy, previous antiretroviral treatment for HIV, and a documented history of poor adherence with HAART, viral failure, and/or treatment discontinuation for more than 1 month out of the 2 years of minimum follow-up.
Demographics, including HIV risk factor, baseline HIV RNA levels, CD4 absolute count, CD4 percentages (%), HCVAb status, and plasma HCV-RNA measurement, were recorded. Patients who had a negative HCV-Ab test or a negative HCV RNA were defined as non-HCV patients. Subsequent CD4 measurements were recorded (absolute counts and CD4%) for each patient at 1 year (+/-2 months) intervals after the baseline measurements up to 3 years after initiation of HAART. We followed the patients for a maximum of 3 years based on previous reports of a plateau in CD4 counts observed after 3 years of HAART.18 The antiretroviral drugs used and changes in regimen performed during the study period were recorded as well. To be included in the analysis, drugs should be present in the regimen for at least 80% of the study period.
Based on a risk of 10%, a power of study of 90%, and an estimated increase in CD4 counts of 150 in the first year, at least 180 patients were calculated to be required. We increased the size of the sample in order to perform stratification
by different factors. Chi-square and Studentfs t tests were used to compare categorical and continuous variables between groups, respectively. Univariate and multivariate linear regression analyses were performed to evaluate predictor factors for CD4 changes in absolute counts and CD4% at three time points: 1 year, 2 years, and 3 years after initiation of antiretroviral therapy. A p value below 0.05 was considered statistically significant. All analyses were performed using the statistical software package SPSS version 10.0 (SPSS Inc., Chicago, IL).
Results
Out of the 322 patients included in the study, 166 had positive HCV-Ab and 156 had negative HCV-Ab. Out of the 166 HCV-Ab positive patients, 27 had undetectable HCV RNA. Therefore, 139 patients were identified as HCV-HIV coinfected and 183 as only-HIV infected. Table 1 summarizes the
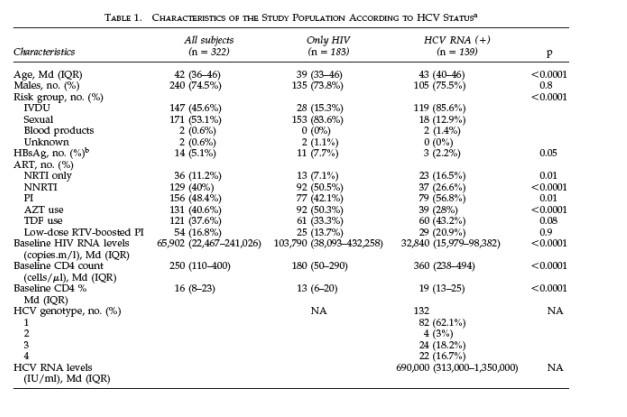
characteristics of the study population according to HCV status. HCV-HIV-coinfected patients were older, more often had a history of intravenous drug use (IVDU), and had less advanced HIV infection at baseline compared to only-HIV patients. The two groups also differed in the type of antiretroviral drugs received. Thus, HCV-HIV-coinfected patients were treated more often with protease inhibitors (PI) or triple nucleoside reverse transcriptase inhibitor (NRTI) regimens and less frequently with nonnucleoside reverse transcriptase inhibitors (NNRTI) compared to only-HIV patients. Three patients took combined didanosine and tenofovir as part of their HAART regimen.
The mean time to follow-up was 34 months, which was comparable for both groups of patients. Changes in the antiretroviral regimen were made in 41% of the patients throughout the study period. For that reason, assessment of
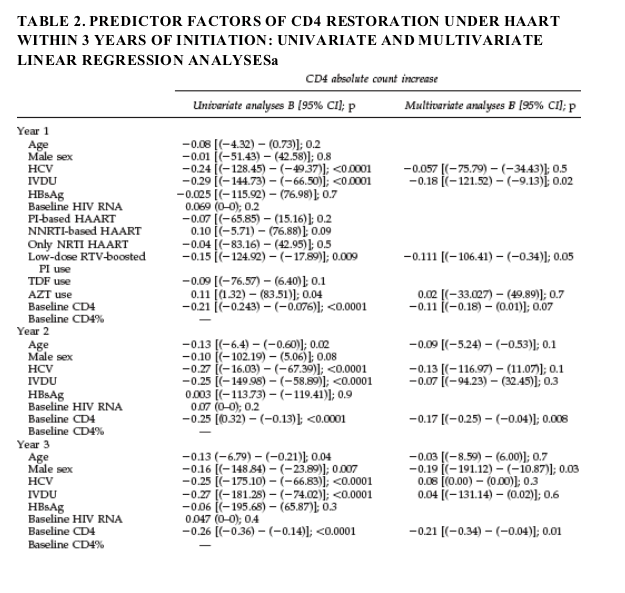
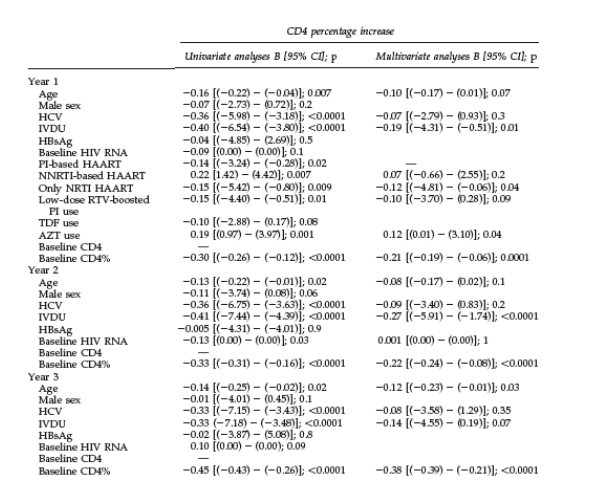
the effect of individual agents or drug families on the CD4 restoration was performed only for the 1-year time point. The results of the univariate and multivariate linear regression analyses evaluating CD4 gains (in absolute counts and in CD4%) at the three different time points are given in Table 2.
A lower baseline CD4 count was the most consistent and strongest factor associated with greater CD4 gains throughout the study period. The analyses of absolute CD4 counts and CD4% as endpoints showed some discordance in the predictor factors identified. HCV coinfection was consistently identified in the univariate analyses as a predictor factor of poorer CD4 restoration at all given time points. However, the association was always lost when other factors were taken into account in multivariate analyses. Among HIVHCV-coinfected patients HCV genotype and HCV replication as continuous variable were also assessed and no associations with CD4 recovery were identified (data not shown).
As displayed in Table 2, at 1 year of HAART, a history of IVDU was associated with smaller increases in absolute CD4 counts [OR -0.20 [95% CI (-128.32)-
(-16.24)]; p =0.01] and in CD4% [OR -0.19 [95% CI (-4.37)-( -0.60)]; p=0.01] in
the multivariate analyses. Higher baseline CD4% [OR 0.18 [95% CI (-0.18)-
(-0.05)]; p =0.001] and use of only NRTI regimens [OR -0.12 [95% CI (-4.81)-
(-0.06)]; p=0.04] were also independent factors predicting poorer gains in
CD4% at 1 year of therapy. However, use of zidovudine (AZT) predicted greater CD4% increases [OR 0.12 [955 CI (0.01)-(3.10)]; p = 0.04].
Poorer absolute CD4 count restoration at 2 years of therapy was found to be associated with higher baseline CD4 counts [OR -0.17 [95% CI ( -0.25)-
( -0.04)]; p =0.008]. Smaller increases in CD4% after 2 years of HAART were associated with higher baseline CD4 percentages [OR -0.22 [95% CI (-0.24)-
(-0.08)]; p <0.0001] and a history of IVDU [OR -0.27 [95% CI ( -5.91)-( -1.74)]; p < 0.0001].
The multivariate analysis evaluating CD4 count restoration after 3 years of therapy identified higher baseline CD4 count [OR -0.21 [95% CI ( -0.34)-
(-0.04)]; p =0.01] and male sex [OR 0.19 [95% CI ( 191.12)-( 10.87)]; p =0.03]
as factors associated with smaller increments in absolute CD4 counts and higher baseline CD4% [OR 0.38 [95% CI ( -0.39)-( -0.21)]; p < 0.0001] and older age [OR 0.12 [95% CI ( -0.23)-( -0.01)]; p = 0.03] as factors predicting smaller increases in CD4%.
Discussion
According to our results HCV coinfection does not impair the CD4 restoration driven by HAART in treatment-naive HIV-infected subjects. While HIV-HCV-coinfected patients showed a poorer CD4 restoration compared to only-HIV patients, the multivariate analyses revealed that other factors and not the HCV infection per se accounted for the differences in CD4 gains between the two cohorts.
Previous studies evaluating the influence of HCV infection on HIV disease progression have reported conflicting results. In the Swiss cohort, HCV-Ab-positive patients had smaller increases in CD4 counts compared to HCV-Ab-negative patients.1 Similar results were found in other European studies.2,5,12 However, other reports have not confirmed those findings.7-11,19,20 A meta-analysis including eight of these studies concluded that HCV/HIV-coinfected subjects might have a less robust increase in CD4 counts beyond 12 months of antiretroviral therapy.21
Most studies assessing the impact of HCV infection on CD4 restoration under HAART have been based on HCV serology. However, some HCV-Ab-positive patients have no HCV replication.22 A small recent study assessing 15 HCV
RNA-positive and 24 only-HIV patients revealed a delay in the CD4 gains in the former group but comparable restoration after 1 year of treatment.23 In our study the same definition for HCV coinfection was used, and with a much larger
sample confirms those findings.
IVDU rather than HCV coinfection has been identified as a factor impairing gains in CD4 counts in HIV-infected individuals under HAART.14,15,24 In line with this, similar CD4 increases after 12 months of HAART were seen in a Thai cohort, with a majority of nonintravenous drug users, regardless of HCV serostatus.10 According to our results, IVDU might delay the restoration of CD4 during the first 2 years of treatment, but seems not to have an impact beyond that time.
The role of plasma HCV-RNA levels and HCV genotype in immune restoration in HIV-infected patients has been evaluated in a few studies, showing conflicting results.12,13,25 Like the authors of two of them, we have not found a correlation between CD4 counts and HCV-RNA levels.12,13 However, our findings do not support previous reports suggesting that HCV-1 is associated with lower CD4 counts.12,13,25
The findings of a smaller increase in the absolute CD4 counts among males compared to females and in CD4% in older compared to younger patients at 3 years of HAART in our study have unclear significance since there was no consistency across endpoints and time points. Comparable CD4 gains according to sex have been reported by other authors.26 As for the effect of age on CD4 recovery, previous studies have shown discordant results, but one of the most comprehensive studies revealed significantly slower CD4 reconstitution in patients above 50 years of age compared to younger patients.27 Another large study showed smaller increases in CD4 counts in older compared to younger patients only during the first year of treatment.28
As for the impact of certain antiretroviral drugs or drug families, PI-based HAART regimens produced higher increases in CD4 counts than NNRT-based regimens in four out of six randomized clinical trials.29-34 Regarding the effect of the NRTI backbone, in a randomized clinical trial in which the third agent was efavirenz, the combination of tenofovir and emtricitabine produced significantly greater increases
in CD4 counts than AZT combined with lamivudine at 1 and 2 years but not at 3 years of follow-up.35-37 Based on those results, we specifically searched for the impact of those drugs on increases in CD4 in the present study, but
our data do not support previous findings. However, we identified weak associations between the use of NRTI-only HAART regimens and of AZT (as part of the NRTI backbone), and poorer and better CD4 restoration, respectively. The significance of these findings is unclear at this time and need to be interpreted with caution.
In summary, HCV replication per se does not impair CD4 restoration in HIV-infected patients successfully treated with antiretroviral therapy. Lower baseline CD4 counts are the strongest predictors of greater CD4 gains over a 3-year period, while a history of IVDU negatively affects CD4 restoration only early after the initiation of therapy.
|
|
| |
| |
|
|
|