| |
Risk for Opportunistic Disease and Death after Reinitiating Continuous Antiretroviral Therapy in Patients with HIV Previously Receiving Episodic Therapy SMART
|
| |
| |
"--Continuous antiretroviral therapy improves outcomes for patients with HIV compared with episodic, CD4+ cell count-guided therapy.
--This long-term follow-up of clinical trial participants demonstrates that the increased hazard of opportunistic disease and death decreases, but is not eliminated, with resumption of continuous antiretroviral therapy in participants initially assigned to episodic therapy.... We attribute the residual excess risk for opportunistic disease or death to lower CD4+ cell counts and higher HIV RNA levels for drug conservation participants compared with viral suppression participants during the post-study modification period.
--An 18-month follow-up may have been too short to assess true changes in hazard.
--Patients who receive episodic antiretroviral therapy decrease but do not eliminate their excess risk for disease when they resume continuous treatment."
In the post-study modification period, the percentage of follow-up time spent with CD4+ cell counts less than 0.350 x 109 cells/L remained greater for the drug conservation group than the viral suppression group (23% vs. 7%).....Similarly, more follow-up time was spent by drug conservation than viral suppression participants with an HIV RNA level greater than 400 copies/mL (40% vs. 17%).
We identified 2 reasons for the persistence of lower CD4+ cell counts and higher HIV RNA levels for drug conservation compared with viral suppression participants during the post-study modification period: Not all participants who ever received antiretroviral therapy reinitiated it, and for those who reinitiated therapy, the return to baseline, preinterruption CD4+ levels was incomplete during the 18-month follow-up.
Before study modification (reinitiating HAART after interruption), more drug conservation participants than viral suppression participants experienced opportunistic disease or cardiovascular, renal, or hepatic events.
Other factors may have contributed to the residual excess risk in the drug conservation compared with the viral suppression group. We recently reported that antiretroviral therapy interruption resulted in increases in interleukin-6 and D-dimer levels, and these biomarkers were associated with increased risk for all-cause mortality (22). It seems that antiretroviral therapy interruption induced activation of tissue factor pathways, thrombosis, and fibrinolysis, and these changes may have long-term effects.
Risk for Opportunistic Disease and Death after Reinitiating Continuous Antiretroviral Therapy in Patients with HIV Previously Receiving Episodic Therapy
A Randomized Trial
SMART Study Group*
Annals of Internal Medicine 2 September 2008 | Volume 149 Issue 5 | Pages 289-299
"Reinitiating continuous antiretroviral therapy in patients previously assigned to episodic treatment reduced excess risk for opportunistic disease or death, but excess risk remained. Episodic antiretroviral therapy, as used in the SMART study, should be avoided."
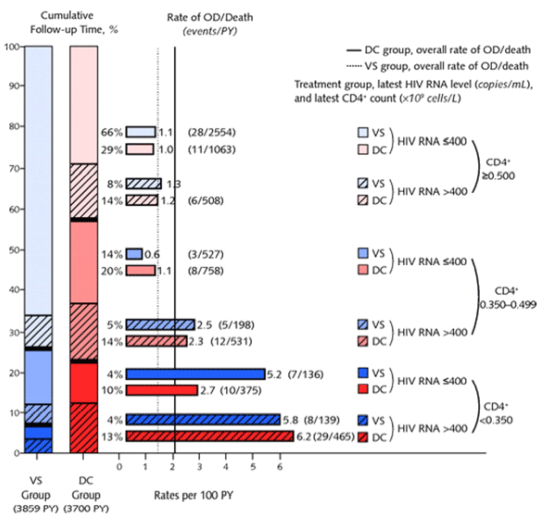
"In both treatment groups, rates of opportunistic disease or death were less for patients with higher levels of latest CD4+ cell count and for those with an HIV RNA level of 400 copies/mL or less (Figure 4). In an analysis pooling the 2 treatment groups, a 100-cell lower latest CD4+ cell count was associated with a 23% (CI, 12% to 35%) higher risk for opportunistic disease or death. An HIV RNA level greater than 400 compared with 400 copies/mL or less was associated with a 92% (CI, 32% to 177%) higher risk for opportunistic disease or death."
"drug conservation participants spent less time than viral suppression participants in CD4+ cell count and HIV RNA strata associated with lower risk for opportunistic disease or death. For example, the percentage of follow-up time after study modification in the lowest risk stratum for opportunistic disease or death (CD4+ cell count ≥0.500 x 109 cells/L and HIV RNA levels ≦400 copies/mL) (Figure 4) was 29% for drug conservation participants and 66% for viral suppression participants."
"Among surviving participants at study modification, 108 in the drug conservation group and 49 in the viral suppression group had experienced an opportunistic disease or a major cardiovascular, renal, or hepatic disease event during the initial follow-up. Participants in both groups with such events were at increased risk for death during the 18 months after study modification. The HR for all-cause mortality for those who experienced at least 1 of these events versus those who did not was 5.8 (CI, 3.2 to 10.7) for both treatment groups combined. With adjustment for the occurrence of an opportunistic disease or a cardiovascular, renal, or hepatic event before study modification, the treatment HR for all-cause mortality after study modification decreased from 1.4 to 1.3"
ABSTRACT
Background: Episodic use of antiretroviral therapy guided by CD4+ cell counts is inferior to continuous antiretroviral therapy.
Objective: To determine whether reinitiating continuous antiretroviral therapy in patients who received episodic treatment reduces excess risk for opportunistic disease or death.
Design: Randomized, controlled trial.
Setting: Sites in 33 countries.
Patients: 5472 HIV-infected individuals with CD4+ cell counts greater than 0.350 x 109 cells/L enrolled from January 2002 to January 2006.
Intervention: Episodic or continuous antiretroviral therapy initially, followed by continuous therapy in participants previously assigned to episodic treatment.
Measurements: Opportunistic disease or death was the primary outcome.
Results:
Eighteen months after the recommendation to reinitiate continuous therapy, mean CD4+ cell counts were 0.152 x 109 cells/L [average of 152 cd4 cells] (95% CI, 0.136 to 0.167 x 109 cells/L) less in participants previously assigned to episodic treatment (P < 0.001), [that is those patients assigned to the treatment interruption arm].
The proportion of follow-up time spent with CD4+ cell counts of 0.500 x 109 cells/L [500] or more and HIV RNA levels of 400 copies/mL or less was 29% for participants initially assigned to episodic therapy and 66% for those assigned to continuous therapy.
Participants who reinitiated continuous therapy experienced rapid suppression of HIV RNA levels (89.7% with HIV RNA levels ≦400 copies/mL after 6 months), but CD4+ cell counts after 6 months remained 0.140 x 109 cells/L [140] below baseline.
The hazard ratio (episodic versus continuous treatment) for opportunistic disease or death decreased after the recommendation to reinitiate continuous therapy (from 2.5 [CI, 1.8 to 3.5] to 1.4 [CI, 1.0 to 2.0]; P = 0.033 for difference). The residual excess risk was attributable to failure to reinitiate therapy by some participants and slow recovery of CD4+ cell counts for those who reinitiated therapy.
Limitation: Follow-up was too short to assess the full effect of switching from episodic to continuous antiretroviral therapy.
Conclusion: Reinitiating continuous antiretroviral therapy in patients previously assigned to episodic treatment reduced excess risk for opportunistic disease or death, but excess risk remained. Episodic antiretroviral therapy, as used in the SMART study, should be avoided.
The SMART (Strategies for Management of Anti-Retroviral Therapy) study was designed to assess whether the risks associated with long-term use of antiretroviral therapy could be reduced through the episodic use of antiretroviral therapy guided by CD4+ cell counts. This treatment interruption strategy (episodic antiretroviral therapy or drug conservation strategy) was compared with the current practice of continuous antiretroviral therapy (viral suppression strategy). As previously reported, the episodic treatment strategy caused an excess risk for opportunistic diseases or death (the primary end point), an excess risk for serious nonopportunistic diseases, and inferior quality of life compared with the continuous antiretroviral therapy strategy (1-4). The excess risk for opportunistic disease or death due to any cause in the drug conservation group was largely attributable to lower CD4+ cell counts and higher HIV RNA levels during follow-up compared with those in the viral suppression group (1, 5). The episodic treatment strategy was discontinued on 11 January 2006, and all participants assigned to the drug conservation group were advised to reinitiate antiretroviral therapy, except for some who had remained antiretroviral therapy-naive. All participants, including those who were still antiretroviral therapy-naive, were then followed for an additional 18 months.
We describe the results of the trial through the end of follow-up and assess the extent to which the excess risk for major clinical outcomes was reduced as a consequence of the recommendation to reinitiate antiretroviral therapy in the drug conservation group.
Design
The design and data collection methods of the trial have been published (1). In brief, 5472 HIV-infected patients with a CD4+ cell count greater than 0.350 x 109 cells/L were randomly assigned at each clinical site to an episodic antiretroviral therapy strategy (drug conservation group) guided by CD4+ cell counts or to continuous antiretroviral therapy (viral suppression group) (Figure 1). The viral suppression strategy aimed to maximally suppress viral replication by continuous use of antiretroviral therapy. The drug conservation strategy entailed episodic use of antiretroviral therapy based on CD4+ cell count thresholds. Therapy was discontinued (or deferred) until CD4+ cell counts decreased to less than 0.250 x 109 cells/L[250], at which time it was reinitiated and continued until the CD4+ cell count increased to greater than 0.350 x 109 cells/L[350]. On confirmation that the CD4+ cell count was greater than 0.350 x 109 cells/L[350], antiretroviral therapy was discontinued and resumed again if the CD4+ cell count returned to less than 0.250 x 109 cells/L[250]. During periods of antiretroviral therapy, the goal was to achieve maximum viral suppression.
Clinical Outcomes
The primary end point was a composite outcome of new or recurrent opportunistic disease or death from any cause, and the secondary end point was a composite of major cardiovascular, renal, and hepatic diseases (1). At the start of the trial, investigators assumed that the risk for cardiovascular, renal, and hepatic diseases was increased with use of antiretroviral therapy. An end point review committee, blinded to treatment group, reviewed opportunistic diseases; deaths; and cardiovascular, hepatic, and renal disease events by using preestablished criteria. Underlying cause of death was classified by using the Coding of Death in HIV Project system (6).
Modification
After 5472 participants had been enrolled and were in follow-up, the study data safety and monitoring board recommended discontinuing enrollment in the trial because of increased risk for opportunistic disease or death in the drug conservation group. On 11 January 2006, investigators and participants were notified of these findings, enrollment was discontinued, and participants in the drug conservation group who had received antiretroviral therapy were advised to restart it (referred to as "study modification"). Follow-up was continued for all participants through 11 July 2007 (study closure), with the goal of assessing the extent of reduction in the excess risk in the drug conservation group that was achieved as a consequence of the recommendation to reinitiate antiretroviral therapy. Participants in the drug conservation group who were treatment-naive at the time of study modification were included in the analysis. With these additional 18 months of follow-up, the study was powered (80%) to detect a hazard ratio (HR) (drug conservation vs. viral suppression) for opportunistic disease or death of 1.8 or more during follow-up.
Results
Participant Characteristics and Follow-up
From 8 January 2002 to 11 January 2006, 5472 participants were randomly assigned at 318 sites in 33 countries-2720 to the drug conservation group and 2752 to the viral suppression group. During this time, approximately 7300 person-years accrued; after study modification, approximately 7700 additional person-years accrued. In total, participants were followed on average for 2.8 years, of which 1.3 years occurred before study modification.
At study modification, 2620 (96.3%) participants in the drug conservation group and 2675 (97.2%) participants in the viral suppression group were alive and still being followed. At study closure, the primary end point status was unknown (could not be verified within 4 months of the closing date) for 106 (3.9%) and 121 (4.4%) participants in the drug conservation and viral suppression groups, respectively (Figure 1).
At baseline, the 2 treatment groups were similar in demographic characteristics (1). At study modification, participants in the drug conservation group were less likely to be prescribed antiretroviral therapy (36% vs. 94%), less likely to have HIV RNA levels of 400 copies/mL or less (35% vs. 82%), and had lower average CD4+ cell counts (0.468 vs. 0.682 x 109 cells/L [95% CI for difference, 0.201 x 109 cells/L to 0.228 x 109 cells/L]). By the end of the study, only 1.8% of drug conservation participants remained antiretroviral therapy-naive (Table 1).
Antiretroviral Therapy Use, CD4+ Cell Counts, and HIV RNA Levels after Study Modification
After the recommendation to initiate antiretroviral therapy in the drug conservation group, use of therapy increased from 35.6% at study modification to 74.2% and 83.4% at 6 and 18 months after study modification (study closure), respectively (Figure 2). Among drug conservation participants who were not receiving therapy at the time of study modification but had previously received antiretroviral therapy, 77.2% were receiving therapy at study closure. For viral suppression participants, 94% were receiving antiretroviral therapy at study modification and 95% were receiving therapy at study closure.
With the increasing use of antiretroviral therapy in the drug conservation group after study modification, the percentage of these participants with HIV RNA levels of 400 copies/mL or less increased (Figure 2, A; Table 2). At study closure, this percentage was similar to that at baseline (71.9% vs. 73.0%) (Table 1). In the viral suppression group, the percentage with HIV RNA levels of 400 copies/mL or less remained stable (Figure 2, A; Table 2). After study modification, the difference between treatment groups in the percentage of participants with HIV RNA levels of 400 copies/mL or less decreased but remained statistically significant at each time point (Table 2).
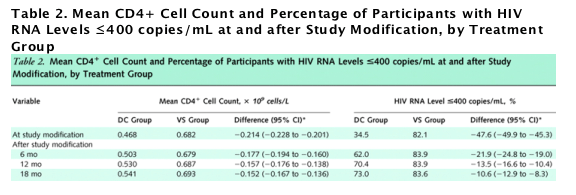
Figure 2. Percentages of participants with HIV RNA levels of 400 copies/mL or less and mean CD4+ cell counts through follow-up.
ART = antiretroviral therapy; DC = drug conservation; VS = viral suppression. A. Percentage of participants with HIV RNA levels ≦400 copies/mL, by treatment group from randomization through the first 4 months and from study modification in January 2006 through study closure. Follow-up time before study modification ranged from 0 to 48 months, depending on the date of enrollment. B. Mean CD4+ cell counts, by treatment group. The gray bars in panels A and B show the percentage of participants in the DC group who received ART. The percentage increased from 35.6% at study modification to 67.0%, 74.2%, 80.5%, and 83.4% at 3, 6, 12, and 18 months after study modification, respectively. The vertical bars show �}2 SEs. C. Percentage with HIV RNA levels ≦ 400 copies/mL among DC participants who received ART at study entry, did not receive ART on 11 January 2006, and restarted ART after study modification. D. Mean CD4+ counts for DC group participants who received ART at study entry, did not receive ART on 11 January 2006, and restarted ART after 11 January 2006.
In the drug conservation group, average CD4+ cell count was 0.120 x 109 cells/L less at study closure than at baseline (Table 1). Average CD4+ cell count increased after study modification for participants in the drug conservation group but remained statistically significantly less than counts for the viral suppression group throughout the 18-month follow-up (Figure 2, B; Table 2).
We investigated whether the CD4+ cell count differences between treatment groups at study closure were because of differences in use of antiretroviral therapy or incomplete recovery of CD4+ cell count in those who reinitiated antiretroviral therapy. There were 974 participants in the drug conservation group who received antiretroviral therapy at study entry, had not received therapy at study modification, and reinitiated therapy after study modification. These participants reinitiated antiretroviral therapy a median of 41 days after study modification (interquartile range, 16 to 100 days). After antiretroviral therapy reinitiation, the percentage who achieved HIV RNA levels of 400 copies/mL or less increased rapidly (Figure 2, C). Six months after reinitiating antiretroviral therapy, the percentage with HIV RNA levels of 400 copies/mL or less was similar to baseline, preinterruption levels (89.7% vs. 88.0%). CD4+ cell counts returned to baseline more slowly (Figure 2, D). Six months after antiretroviral therapy was reinitiated, CD4+ cell count increased an average of 0.151 x 109 cells/L (CI, 0.138 to 0.164 x 109 cells/L); the CD4+ cell count at 6 months after antiretroviral therapy reinitiation, however, remained 0.140 x 109 cells/L (CI, 0.123 to 0.157 x 109 cells/L) below baseline (preinterruption) levels (Figure 2, D).
Before study modification, participants in the drug conservation group had CD4+ cell counts less than 0.350 x 109 cells/L for 31% of the follow-up; after study modification, this percentage decreased to 23%. During both periods, viral suppression participants had CD4+ cell counts less than 0.350 x 109 cells/L for less than 10% of the follow-up. Also, before study modification, participants in the drug conservation group had HIV RNA levels greater than 400 copies/mL for 71% of the follow-up; this decreased to 40% after study modification. For participants in the viral suppression group, this percentage also decreased, from 28% before to 17% after study modification.
Primary End Point and Major Secondary End Points
The rate of first occurrence of opportunistic disease or death decreased by 38% in the drug conservation group after study modification (from 3.4 to 2.1 events per 100 person-years), whereas the rates in the viral suppression group were similar for the 2 periods (1.4 events per 100 person-years) (Table 3). The HR for the study's primary outcome decreased from 2.5 (CI, 1.8 to 3.5; P <0.001) before to 1.4 (CI, 1.0 to 2.0; P = 0.039) after study modification (P = 0.033 for difference) (Table 3). These estimates were not substantively altered in an analysis stratified by site (data not shown). Kaplan-Meier curves for the 2 time periods (through the first 18 months after randomization censored at the date of study modification [Figure 3, top] and through 18 months after study modification [Figure 3, bottom]) illustrate a decrease in the excess risk in the drug conservation group after study modification. The treatment HR for opportunistic disease or death decreased in the period after study modification, but the trend was not statistically significant (P = 0.29) (Figure 3, bottom).
Figure 3. Kaplan-Meier curves for the cumulative probability of opportunistic disease or death from any cause before (top) and after (bottom) study modification.
Estimated cumulative probabilities of the primary end point for the first 18 months after randomization, censored at study modification (top). Cumulative probabilities from study modification to study closure 18 months later; participants who experienced a primary event before study modification were excluded (bottom). DC = drug conservation; HR = hazard ratio; VS = viral suppression.* Censored at the date of study modification.{dagger} After study modification.
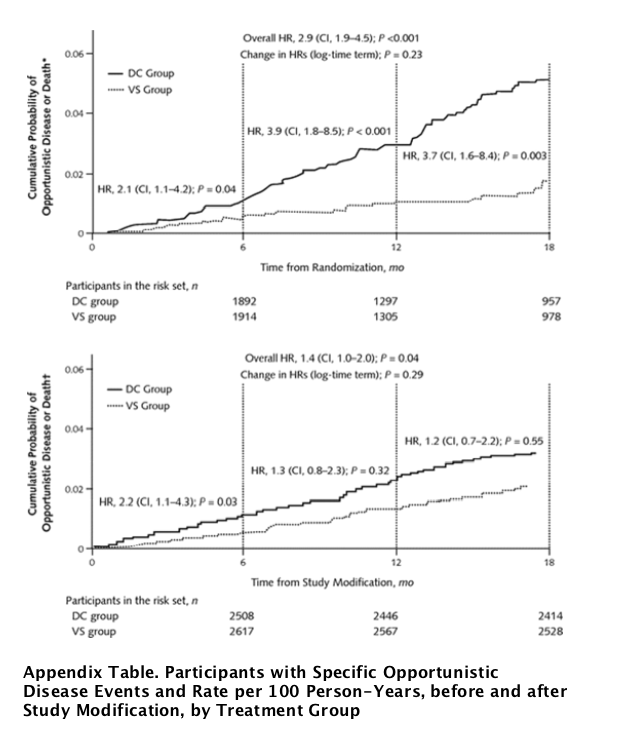
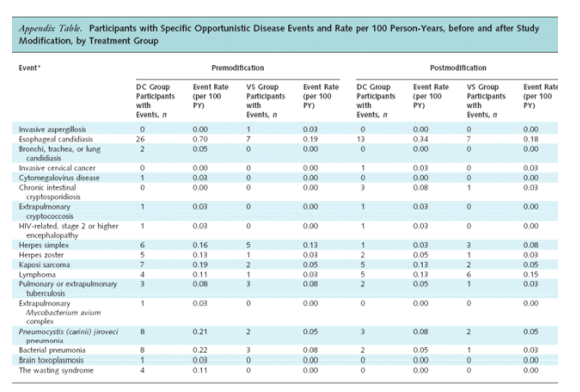
The decrease in the rate of opportunistic disease or death in the drug conservation group, compared with the rate before study modification, was primarily due to the substantial decrease in the rate of opportunistic disease (2.1 events per 100 person-years before vs. 1.0 event per 100 person-years after) (Table 3). The treatment HR for opportunistic disease (fatal or nonfatal) decreased from 3.3 (CI, 2.1 to 5.2; P < 0.001) to 1.7 (CI, 1.0 to 2.9; P = 0.039) after study modification (P = 0.100 for difference).
The treatment HR for all-cause mortality decreased from 1.8 (CI, 1.2 to 2.9; P = 0.007) to 1.4 (CI, 0.9 to 2.2; P = 0.102) after study modification (P = 0.44 for difference) (Table 3). Only 7 of the 82 deaths that occurred after study modification were attributed to opportunistic disease, and all 7 occurred among participants in the drug conservation group. The end point review committee classified 9 deaths in the drug conservation group and 11 deaths in the viral suppression group as "unknown cause." Of these deaths with unknown cause, 5 in each group were unwitnessed. For the drug conservation group, the most frequent causes of death were cardiovascular disease (n = 9) and opportunistic disease (n = 7); for the viral suppression group, the most frequent cause was malignant conditions that were not considered opportunistic (n = 8).
Among surviving participants at study modification, 108 in the drug conservation group and 49 in the viral suppression group had experienced an opportunistic disease or a major cardiovascular, renal, or hepatic disease event during the initial follow-up. Participants in both groups with such events were at increased risk for death during the 18 months after study modification. The HR for all-cause mortality for those who experienced at least 1 of these events versus those who did not was 5.8 (CI, 3.2 to 10.7) for both treatment groups combined. With adjustment for the occurrence of an opportunistic disease or a cardiovascular, renal, or hepatic event before study modification, the treatment HR for all-cause mortality after study modification decreased from 1.4 to 1.3 (CI, 0.8 to 2.1).
The rate of cardiovascular, renal, or hepatic disease decreased by 39% for participants in the drug conservation group. The treatment HR for major cardiovascular, renal, or hepatic disease decreased from 1.7 (CI, 1.1 to 2.5; P = 0.009) to 1.2 (CI, 0.7 to 1.8; P = 0.49) (P = 0.23 for difference) (Table 3). Findings for the outcomes in Table 3 were similar for the large subgroup of patients who received antiretroviral therapy at entry (data not shown).
Rate of Primary End Point, by Latest CD4+ Cell Count and HIV RNA Level
In both treatment groups, rates of opportunistic disease or death were less for patients with higher levels of latest CD4+ cell count and for those with an HIV RNA level of 400 copies/mL or less (Figure 4). In an analysis pooling the 2 treatment groups, a 100-cell lower latest CD4+ cell count was associated with a 23% (CI, 12% to 35%) higher risk for opportunistic disease or death. An HIV RNA level greater than 400 compared with 400 copies/mL or less was associated with a 92% (CI, 32% to 177%) higher risk for opportunistic disease or death. When the rates of opportunistic disease or death were considered along with the amount of time in each stratum, drug conservation participants spent less time than viral suppression participants in CD4+ cell count and HIV RNA strata associated with lower risk for opportunistic disease or death. For example, the percentage of follow-up time after study modification in the lowest risk stratum for opportunistic disease or death (CD4+ cell count ≥0.500 x 109 cells/L and HIV RNA levels ≦400 copies/mL) (Figure 4) was 29% for drug conservation participants and 66% for viral suppression participants. We previously reported that adjustment for differences in latest levels of CD4+ cell count and HIV RNA levels between the drug conservation and viral suppression groups explained a large fraction of the excess risk for opportunistic disease or death in the period before study modification (1, 5). Similar analyses were conducted for the period after study modification. The treatment HR for opportunistic disease or death decreased from 1.4 (CI, 1.0 to 2.0) to 0.9 (CI, 0.6 to 1.3) after adjustment for both latest CD4+ cell count and HIV RNA level.
Figure 4. Percentages of follow-up time spent in categories by latest CD4+ cell counts and latest HIV RNA levels and rates of opportunistic disease or death during this time.
Stacked vertical bars on the left show the percentage of follow-up time spent in each of the 6 categories, by latest CD4+ counts <0.350, 0.350 to 0.499, and ≥0.500 x 109 cells/L and latest HIV RNA levels >400 and ≦400 copies/mL for participants in the drug conservation (DC) group (solid line) and viral suppression (VS) group (dotted line). Percentage of follow-up time appears to the right of the stacked vertical bars. Horizontal bars show the rates of opportunistic disease (OD) or death during follow-up spent at the latest CD4+ and HIV RNA levels in the DC and VS groups. Numbers to the right of the bars are the rates per 100 person-years (PY) (number of events, number of PY).
DISCUSSION
As a consequence of the recommendation to initiate antiretroviral therapy for treatment-experienced participants in the drug conservation group, excess risk for opportunistic disease or death statistically significantly decreased during the 18 months after study modification compared with the period before study modification. Treatment HRs for other major outcomes also decreased. However, residual excess risk remained at study closure. We attribute the residual excess risk for opportunistic disease or death to lower CD4+ cell counts and higher HIV RNA levels for drug conservation participants compared with viral suppression participants during the post-study modification period.
In the post-study modification period, the percentage of follow-up time spent with CD4+ cell counts less than 0.350 x 109 cells/L remained greater for the drug conservation group than the viral suppression group (23% vs. 7%). Similarly, more follow-up time was spent by drug conservation than viral suppression participants with an HIV RNA level greater than 400 copies/mL (40% vs. 17%). Previous trial data before study modification and data from other studies indicate that these differences would be expected to result in a continued higher risk for opportunistic disease or death in the drug conservation group than in the viral suppression group (5, 7-11). Consistent with these observations, adjustment for the latest CD4+ cell counts and HIV RNA levels explains much of the difference in opportunistic disease or death between the 2 treatment groups. Higher rates of nonopportunistic disease mortality would also be predicted by these CD4+ cell count and HIV RNA level differences (12, 13). The decreasing treatment HR for opportunistic disease or death (Figure 3, bottom) after study modification is also consistent with the decreasing differences in CD4+ cell count and percentage with HIV RNA levels of 400 copies/mL or less during the 18 months after study modification (Figure 2, A and B; Table 2).
We identified 2 reasons for the persistence of lower CD4+ cell counts and higher HIV RNA levels for drug conservation compared with viral suppression participants during the post-study modification period: Not all participants who ever received antiretroviral therapy reinitiated it, and for those who reinitiated therapy, the return to baseline, preinterruption CD4+ levels was incomplete during the 18-month follow-up. Drug conservation participants who did not reinitiate antiretroviral therapy after the study modification had higher nadir, baseline, and study modification CD4+ cell counts (0.400, 0.816, and 0.623 x 109 cells/L, respectively) than did those who reinitiated after study modification (0.262, 0.668, and 0.458 x 109 cells/L, respectively). Perhaps participants and clinicians may have felt that they were "safe" on the basis of their previous and current counts.
Although the percentage of drug conservation participants who achieved HIV RNA levels of 400 copies/mL or less after reinitiating antiretroviral therapy rapidly increased, CD4+ cell counts increased more gradually. Other interruption studies have reported CD4+ cell counts that do not rapidly return to preinterruption levels (14-16). The rate of CD4+ cell count recovery seen over 6 to 18 months (Figure 2, D) followed a characteristic 2-phase increase within the numeric range reported for HIV-infected patients on initiation of treatment in large antiretroviral therapy-naive cohorts (17-19) and among viral suppression participants who initiated antiretroviral therapy after randomization (20). This pattern of CD4+ cell count recovery indicates that more than 18 months, the maximum follow-up time after study modification, would have been required for the average CD4+ cell count to return to preinterruption levels (18, 21).
Before study modification, more drug conservation participants than viral suppression participants experienced opportunistic disease or cardiovascular, renal, or hepatic events. Risk for death after study modification was statistically significantly greater among participants who experienced 1 of these events than among those who did not. These observations may help explain the mortality rate differences between the treatment groups during the 18 months after study modification.
Other factors may have contributed to the residual excess risk in the drug conservation compared with the viral suppression group. We recently reported that antiretroviral therapy interruption resulted in increases in interleukin-6 and D-dimer levels, and these biomarkers were associated with increased risk for all-cause mortality (22). It seems that antiretroviral therapy interruption induced activation of tissue factor pathways, thrombosis, and fibrinolysis, and these changes may have long-term effects.
Our findings emphasize the importance of continued follow-up of participants in trials after identifying deleterious effects of 1 of the interventions and making protocol changes. However, a limitation of our study is that follow-up was too short to assess the full effect of switching from episodic to continuous antiretroviral therapy. As a consequence, although we have good power for confirming a decrease in risk for opportunistic disease or death, power was lower for other outcomes (for example, death and major cardiovascular, renal, or liver diseases), for which fewer events occurred and HRs were not as large before the study modification.
In conclusion, the recommendation to reinitiate antiretroviral therapy resulted in a 38% decrease in the rate of opportunistic disease or death in the drug conservation group. Compared with the viral suppression group, an ongoing though diminished excess risk for opportunistic disease or death remained during the 18 months after this recommendation. Several factors contribute to this excess risk. It seems that our follow-up was too short to observe full reversal of risk, even among those who initiated antiretroviral therapy, as recommended. These findings reinforce the recommendation to avoid use of episodic antiretroviral therapy guided by CD4+ cell counts used in the trial.
|
|
| |
| |
|
|
|