| |
The relationship between an adverse effect of cART and underlying risk illustrated by number needed to treat to harm (NNTH) - Risk of myocardial infarction and abacavir use
|
| |
| |
Reported by Jules Levin
Justyna D. Kowalska, Ole Kirk, Amanda Mocroft, Leif Hoj, Nina Friis-
Moller, Peter Reiss, Jens D. Lundgren
Copenhagen HIV Program (CHIP), University of Copenhagen, Denmark
Conclusions
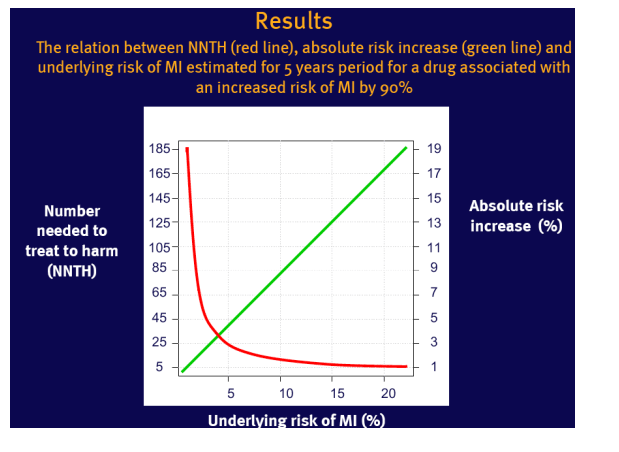
NNTH carries different information than absolute risk increase
There is a reciprocal relation between the risk of adverse effect of treatment presented in NNTH and the underlying risk of this condition
This is true for any condition with underlying risk variation
Clinical implications
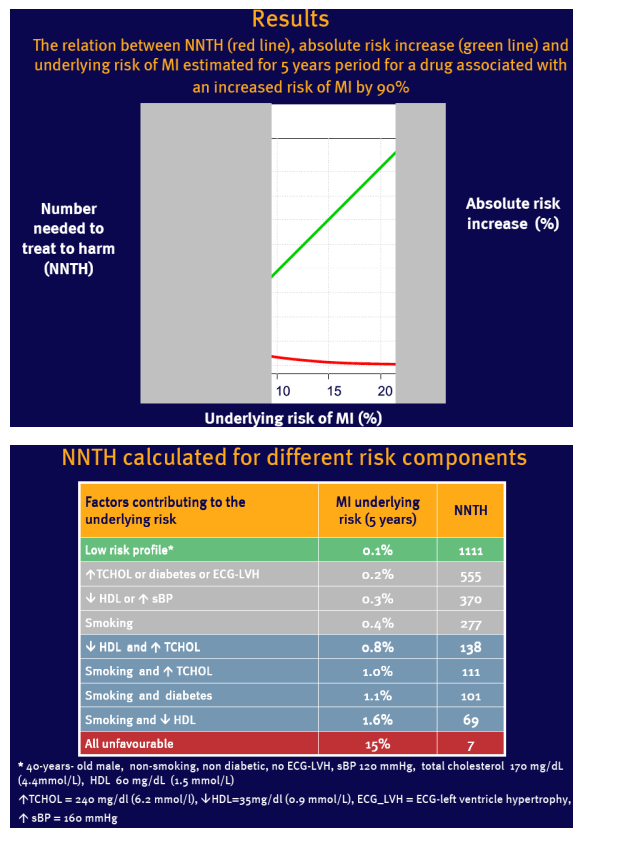
It is possible to increase NNTH values for any patient on abacavir by decreasing the underlying risk of MI
Individual assessment of underlying risk may play an important role in decreasing the risk of adverse effect of cART
Rationale for the study
With potentially life-long treatment it is crucial to ensure cART adverse effects are reduced as much as possible
There is no standard approach on how to assess individual risk of adverse effects of cART
It has not been investigated whether and to what extent lowering the underlying risk may influence the size of adverse effect
Aim of the study
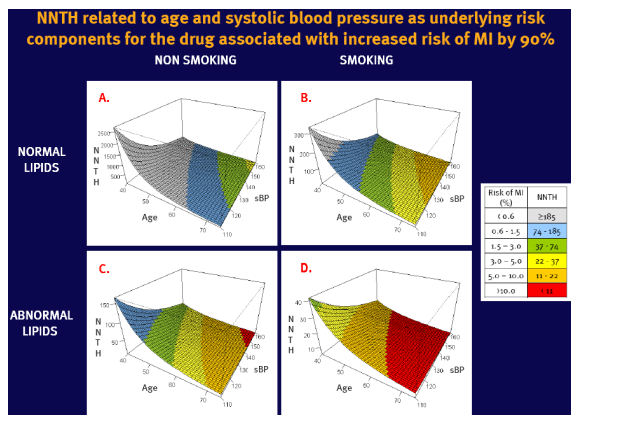
To investigate relationship between an adverse event of cART and the underlying risk of this event, and illustrate it by number needed to treat to harm (NNTH)
To present results in easy to understand, graphical form
What is the number needed to treat to harm (NNTH)?
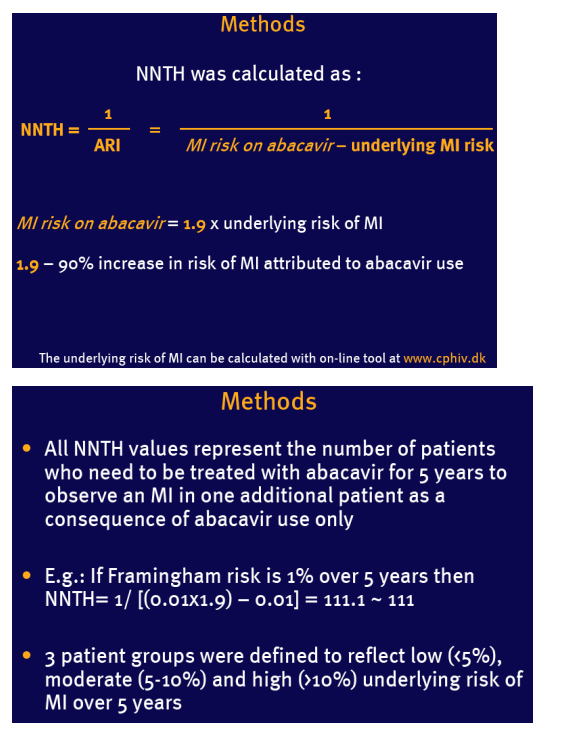
NNTH is a measure of negative treatment effect and is calculated as 1/absolute risk increase (ARI)
The higher NNTH, the better
NNTH of 10 means that we need to treat 10 patients to observe an adverse treatment effect in one additional patient
NNTH is strongly supported by methodological papers, but its use in practice is uncommon
Why NNTH is not widely used?
A number of factors need to be considered in interpretation:
⋅ size of negative effect
⋅ specific context
⋅ delay in adverse effect onset
No pre-defined NNTH cut-offs for what is acceptable or not
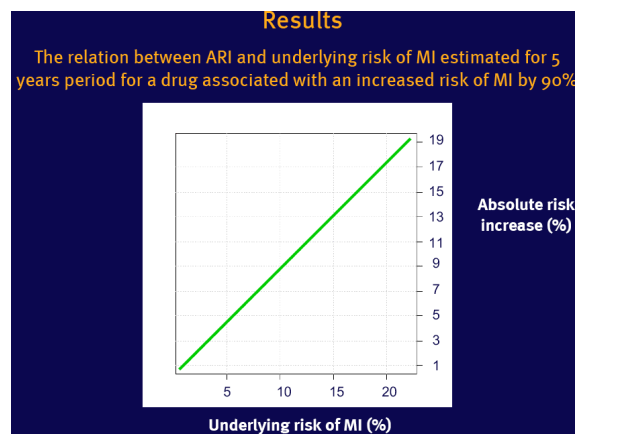
NNTH varies with the underlying risk, which limits its generalisability for a population with large risk variation
Methods
Example used: a 90% increased relative risk (RR=1.9) of myocardial infarction (MI) in patients on abacavir reported recently by the D:A:D study group (1,2)
Assumptions: RR remains constant across the range of underlying risk of MI
Underlying risk of MI was estimated for 5 years based on the Framingham Heart Study population (3)
1. The D:A:D Study Group. Lancet 2008
2. SMART/Insight and D:A:D Study Group. AIDS 2008
3. Anderson KM. Am Heart J. 1991
|
|
| |
| |
|
|
|