 |
 |
 |
| |
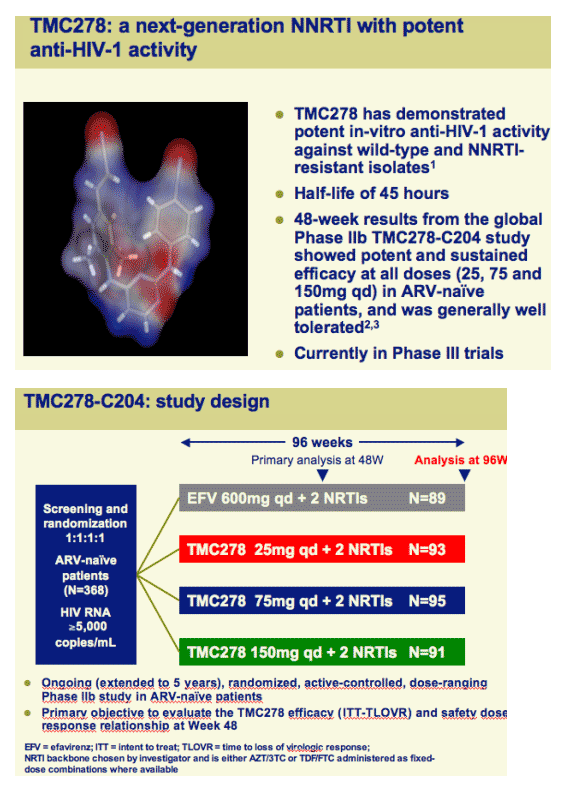
TMC278 (rilpivirine), an investigational next-generation NNRTI, demonstrates long-term efficacy and tolerability in ARV-naive patients: 96-week results of study C204
|
| |
| |
Reported by Jules Levin
M Santoscoy,1 P Cahn,2 C Gonsalez,3 W Hao,4
A Pozniak,5 P Shalit,6 S Vanveggel,7 K Boven8
1Hospital Carlos Mac Gregor IMSS, Mexico City, Mexico; 2Hospital Juan A Fernández and Fundación Huesped, Buenos Aires, Argentina; 3Hospital das Clíncias, Pinheiros, Brazil; 4Beijing You’an Hospital, Beijing, China; 5Chelsea and Westminster NHS Foundation Trust and PKR/SSR, London, UK; 6Swedish Medical Center, Seattle, WA, USA; 7Tibotec BVBA, Mechelen, Belgium; 8Tibotec Inc., Yardley, PA, USA.
AUTHOR CONCLUSIONS
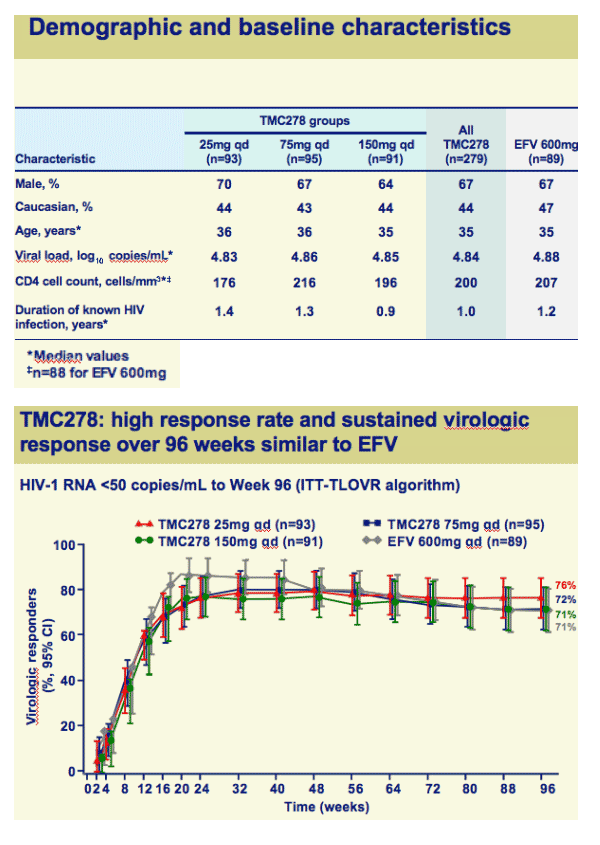
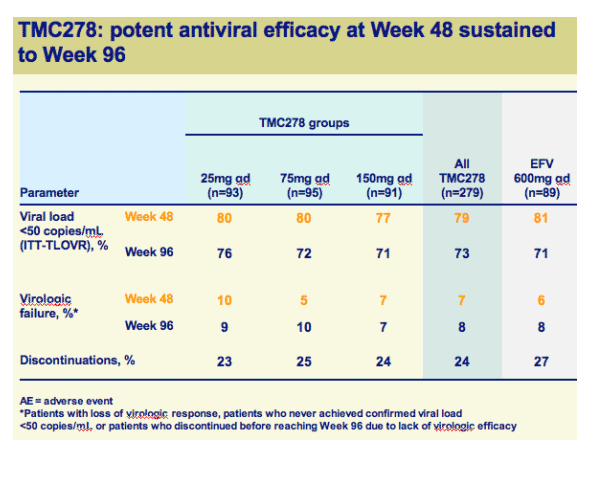
Once-daily oral TMC278 at all doses demonstrated a high response rate and sustained virologic response over 96 weeks
TMC278 was generally safe and well tolerated
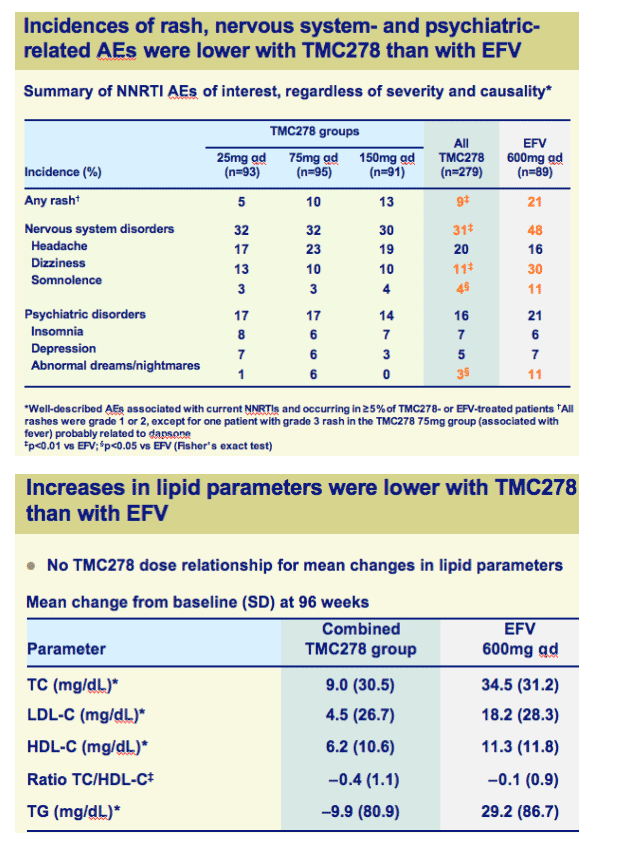
Incidences of rash, nervous system- and psychiatric-related AEs and increase in lipids were significantly lower with TMC278 than with EFV
There were trends suggesting a favourable profile of TMC278 25mg compared with the higher dose groups
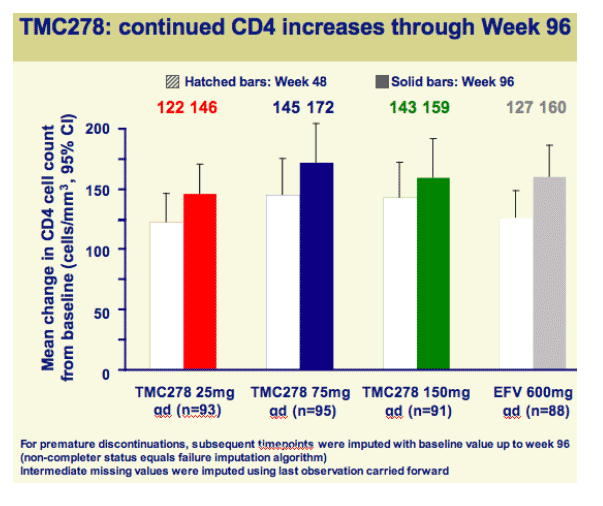
Both efficacy and safety of TMC278 were well maintained between 48 and 96 weeks
TMC278 is being further evaluated in Phase III trials at a dose of 25mg qd
|
| |
|
 |
 |
|
|