 |
 |
 |
| |
Efavirenz induces alterations in lipid metabolism through AMPK activation
|
| |
| |
Reported by Jules Levin
10th Intl Workshop on Adverse Drug Reactions and Lipodystrophy in HIV, London Nov 6-8 2008
Ana Blas-García, Daniel Ballesteros, Daniel Monleón, José M. Morales, Milagros Rocha, Víctor M. Víctor, Nadezda Apostolova and Juan V. Esplugues.1 Departamento de Farmacología (Facultad de Medicina) y 2 Hospital Universitario Dr. Peset, Universidad de Valencia y CIBERehd (Spain)
INTRODUCTION
Lipodystrophy and associated metabolic alterations are the most prevalent adverse effects in patients taking HAART, but the specific drugs responsible and mechanisms involved in its appearance??? are still unclear.
Recent clinical evidence suggests that the NNRTI Efavirenz (EFV) contributes to changes in lipid composition. As liver has a major role in general metabolism we have evaluated the acute effect of this NNRTI on several mitochondrial parameters, in order to determine an acute effect of this drug on the mitochondria, independent of any effect on mtDNA, which could result in changes in important cellular bioenergetic parameters.
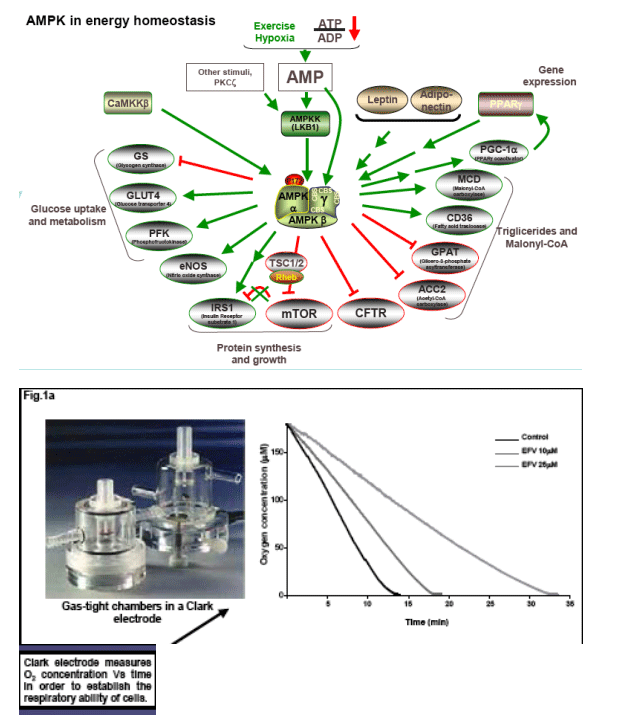
AMP-activated protein kinase (AMPK) is a master switch for regulation of cellular energetics in health and disease. AMPK is activated by various forms of stress that compromise cellular ATP levels and, once phosphorylated, AMPk-P promotes catabolic pathways to restore energy homeostasis. This response varies depending on the implicated organ; short-term activation in the liver results in a switch from glucose to fatty acid utilization to supply energy needs.
Inhibiton of mitochondrial respiration could lead to activation of AMPK and, thus, to changes in lipid metabolism.
AUTHOR CONCLUSIONS
Clinically used concentrations of EFV acutely reduce mitochondrial function in Hep3B, as suggested by the inhibition of O2 consumption, and the decrease of Δ&968;m.
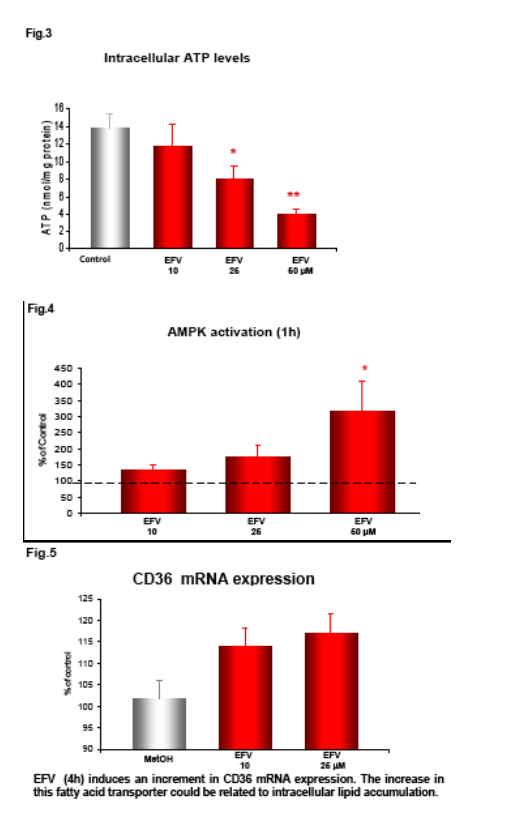
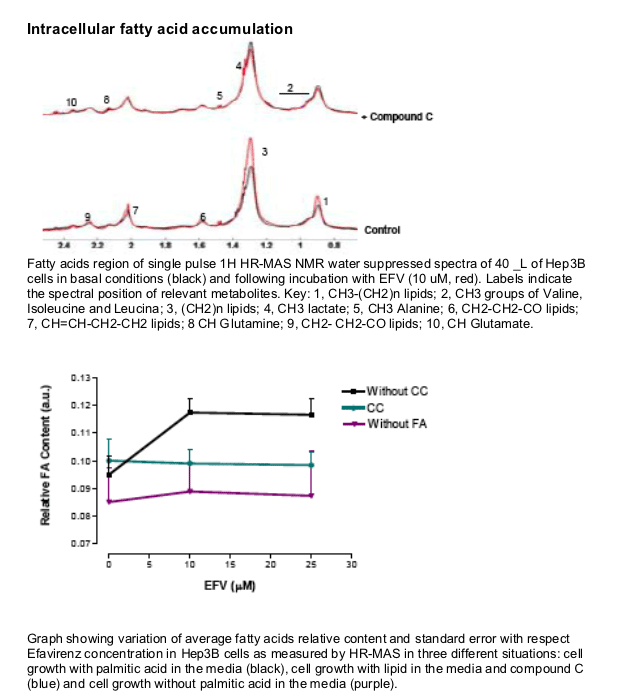
EFV induces a reduction in ATP levels, leading to a bioenergetic stress as shown by the activation of AMP-activated protein kinase, and upregulates CD36 mRNA expression, thus facilitating fatty acids uptake. This drug also induces intracellular lipid content, which could have been a result of the formation of lipid droplets. This intracellular lipid increase was not present in cells treated with Compound C, suggesting a key role of AMPK in this processes.
Given that EFV treatment is usually prolonged, these mechanisms may effect the
general regulation of lipid metabolism and could cause the alterations that are characteristic of lipoatrophy.
From Jules: in the abstract the authors say: given that EFV treatment is usually prolonged, these mechanisms might affect the general regulation of lipid metabolism and could cause the alterations that are characteristic of lipoatrophy. So, are these methods good science and are the findings reliable???
METHODS & MATERIALS
Non HIV infected Hep3B cells, cultured in Minimum Essential Medium, were placed in gas-tight chambers and O2 consumption was measured with a Clark-type O2 electrode after addition of Efavirenz (EFV).
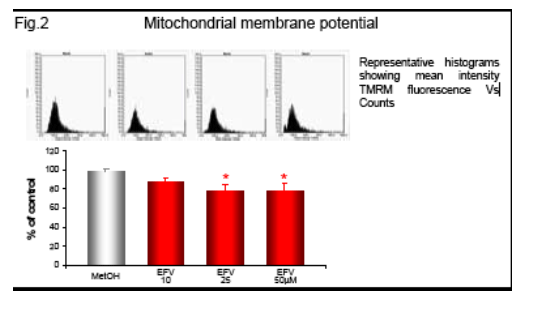
Following incubation with EFV (1h) the intracellular ATP was measured by fluorescence (ATP Bioluminiscence Assay Kit HSII, Roche), and mitochondrial membrane potential (Δ&968;m), indicative of mitochondrial function, was analyzed by static cytometry with a fluorescence microscope (Olympus Ix81). Phosphorylation of AMP-activated protein kinase (AMPk) after 1h incubation with EFV was evaluated by Western blot and quantified by densitometry. mRNA expression of the fatty acid transporter CD36 was anlyzed by RT-PCR and quantifed also by densitometry.
Intracellular lipid content following 4h treatment with EFV was determined by Nuclear Magnetic Resonance.
Data (n≥3) were analyzed using one-way ANOVA. * p<0.05, ** p<0.01, *** p<0.001 (vs control)
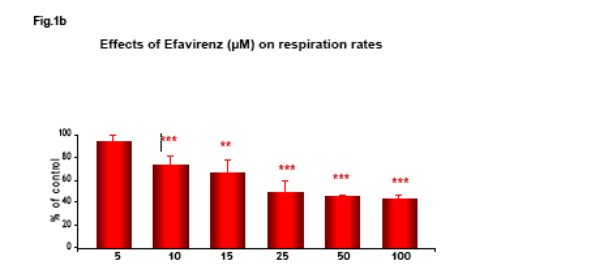
Efavirenz induces a significant and dose-dependent inhibition of mitochondrial O2 consumption (Fig.1b), which was noticeable immediately after addition of the drug (Fig.1a). Incubation with EFV (1h) produces a decrease in mitochondrial membrane potential (Δ&968;m) and a dose-dependent reduction of intracellular ATP (Fig. 2-3).
REFERENCES
Foretz M. et al. Diabetes, 54: 1331-1339 (2005)
Hardie D.G. Nat Rev Mol Cell Biol, 8: 774-785 (2007).
\Modica-Napolitano J.S. Biochem Biophys Res Commun, 194:170-177 (1993).
Petit F. et al. Trends Pharm Sci, 26: 258-264 (2005).
Villarroya F. et al. Trends Pharm Sci, 26: 88-93 (2005).
|
| |
|
 |
 |
|
|