 |
 |
 |
| |
In Vitro Studies Demonstrate that Combinations of Antiviral Agents that Include HCV Polymerase Inhibitor ANA598 have the Potential to Overcome Viral Resistance
|
| |
| |
Reported by Jules Levin
AASLD San Francisco Nov 2008
Peggy A. Thompson, Rupal A. Patel, Richard E. Showalter, Cindy Li, James R. Appleman, and Kevin R. Steffy
Anadys Pharmaceuticals, Inc. San Diego, CA 92121 USA
AUTHOR CONCLUSIONS
ANA598 is a highly potent non-nucleoside inhibitor of HCV genotypes 1b and 1a NS5B polymerases (3 and ∼50 nM, respectively)
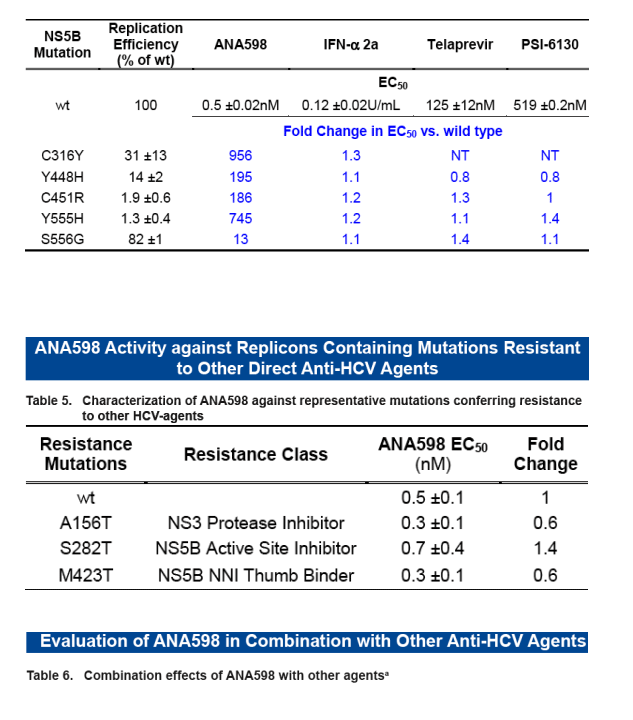
Interferon-a 2a and direct antiviral agents retain potency against mutations selected by ANA598
Activity of ANA598 is retained against replicons containing mutations that confer resis-tance to other direct anti-HCV agents
Minimal cross resistance observed for inhibitors binding at different sites
Additivity and/or synergy was observed in in vitro combination studies of ANA598 with other antiviral agents
In summary, preclinical in vitro results suggest the potential to construct useful treat-ments combining ANA598 with other anti-HCV agents, including ANA773
ABSTRACT (revised)
ANA598 is a novel HCV non-nucleoside polymerase inhibitor that is in clinical develop-ment for the treatment of hepatitis C. Due to the high potential for developing resistance to any direct antiviral used as monotherapy in hepatitis C, it is believed that optimal use of direct antivirals will be in combination regimens designed to minimize emergence of resis-tance. Therefore, it is important to assess combinations of agents for absence of antago-nism, characterize in vitro patterns of resistance to new direct antivirals, identify agents which retain full potency to the characterized mutations, and determine if new antivirals possess activity against mutations known to confer resistance to other agents. This infor-mation will enable future clinical exploration of optimized combination regimens.
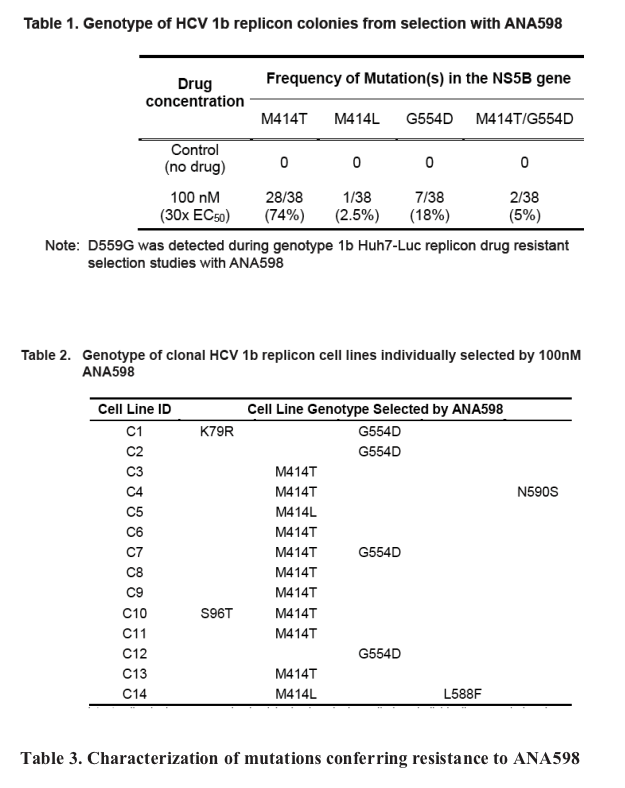
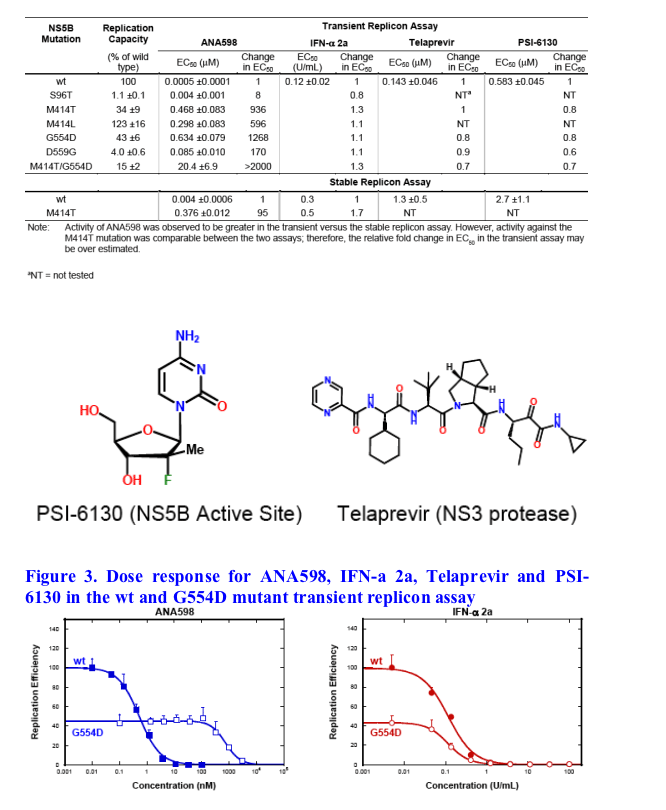
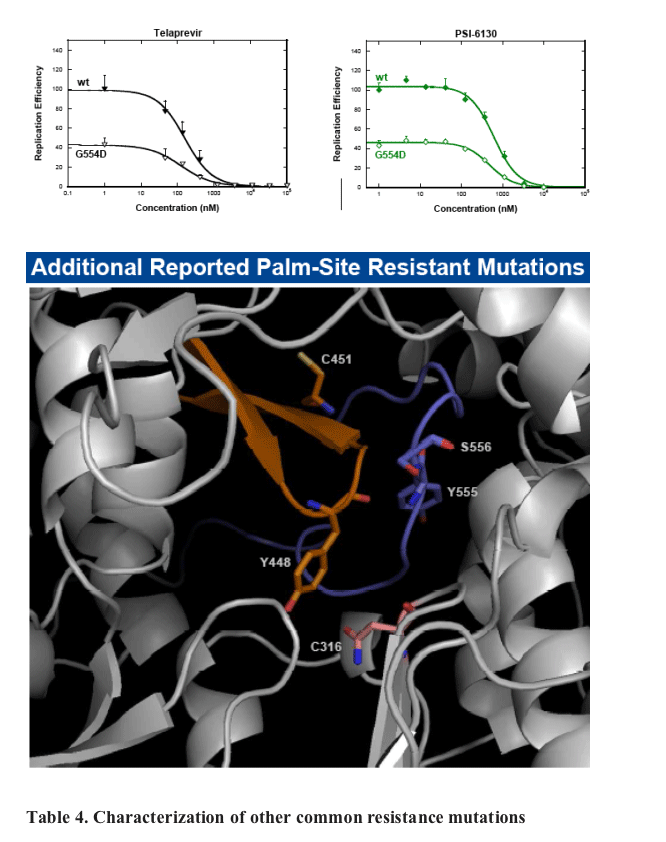
ANA598 exhibits nanomolar potency against genotype 1 HCV replicons (EC50 1b 3nM; 1a 50nM). We utilized in vitro assays to explore the potential of combining ANA598 with other anti-HCV agents, including current standard of care (SOC) as well as direct antivi-rals currently in development. ANA598 was shown to be synergistic and/or additive with IFN-a (wt and M414T mutant replicons), Telaprevir, PSI-6130, and ANA773. Ribavirin was not antagonistic with ANA598. No change in susceptibility was observed when ANA598 was tested with replicons containing the primary mutations known to confer resistance to other direct antivirals including A156T (NS3 protease active site), S282T (NS5B active site) and M423T (NS5B non-nucleoside thumb binding site). Genotypic mutations resis-tant to ANA598 (M414T, M414L, G554D, D559G and M414T/G554D) were identified in vitro. Reduced potency was observed for ANA598 against all mutations with EC50 val-ues of 0.47, 0.30, 0.63, 0.085 and 20mM determined for M414T, M414L, G554D, D559G and M414T/G554D, respectively. Replicons containing mutations that confer resistance to ANA598 remained fully susceptible to IFN-a, Telaprevir (NS3 protease inhibitor), and PSI-6130 (nucleoside polymerase inhibitor).
BACKGROUND
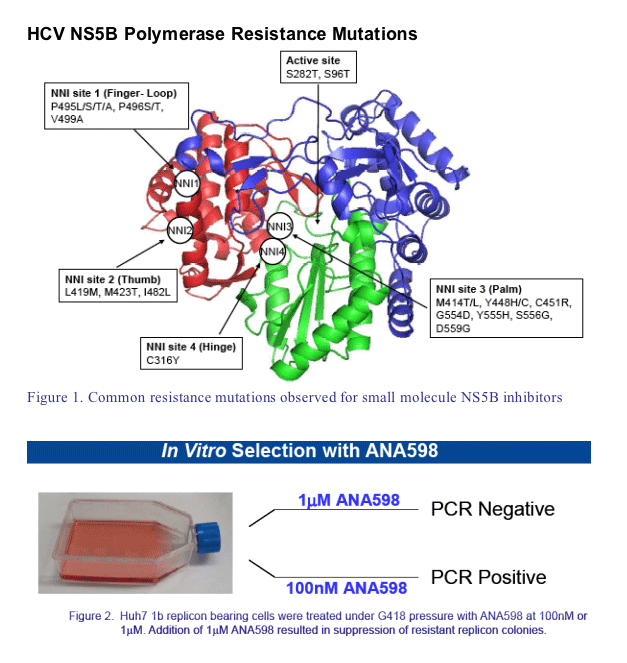
ANA598 is a non-nucleoside polymerase inhibitor in clinical development for the treat-ment of hepatitis C
ANA598 binds to NS5B 1b with subnanomolar potency (KD ∼0.7nM) and dissociates slowly from the enzyme with a t1/2 ∼2 hours
ANA598 exhibits nanomolar potency in HCV replicon assays with EC50 (1b) ∼3nM and EC50 (1a) ∼50nM
ANA598 exhibits no appreciable cellular cytotoxicity in the Huh-7 cell line as indicated by an XTT CC50 >100mM and cellular GAPDH EC50 >100mM
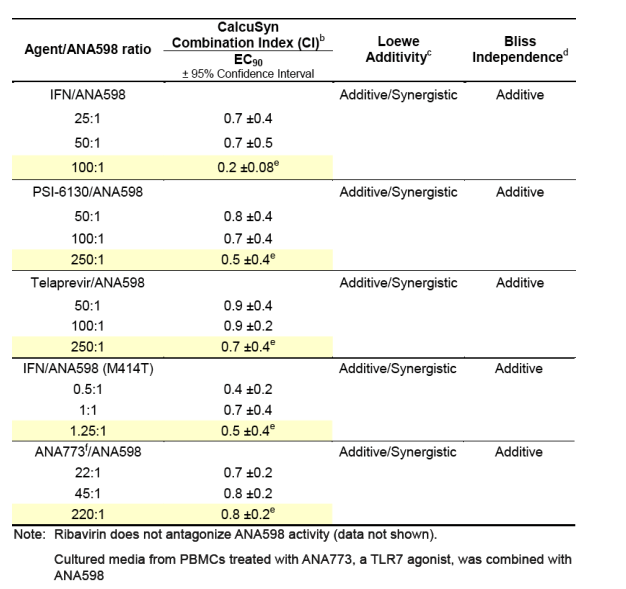
aCombination studies were performed in wild-type Huh7-Luc 1b replicon cells or the 1b dicistronic replicon cells containing mutation M414T. Cells were cultured in the presence of compound for 72 hours. The ratio of the two agents either remained fixed or was varied across the dosing range to explore the greatest combination surface. No cytotoxicty was observed for the concentration range evaluated. Data were analyzed by three mathematical analysis tools.
bCI values between 0.90 and 1.10 = additive; <0.90 = synergy; >1.10 = antagonism
cDegree of additivity or synergy is dependent on the region of the surface
analyzed
dMajority of surface was consistent with Bliss additivity
eFixed concentration ratios highlighted in yellow are conditions where the initial concentrations of both agents are 10-fold above their EC50 values.
fANA773 is an oral prodrug of a potent, selective small-molecule TLR7 agonist discovered at Anadys Pharmaceuticals that is in clinical development for the treatment of patients chronically infected with HCV and for patients with cancer. In vivo and in vitro studies have shown that the active metabolite of ANA773 induces multiple subtypes of interferon-alpha (IFN-a) in human peripheral blood mononuclear cells (PBMCs), and the conditioned media from PBMCs inhibits HCV replication. Conditioned PBMC media from multiple donors were pooled after being exposed to 100mM of the active metabolite of ANA773 and used in the combination studies.
|
| |
|
 |
 |
|
|