 |
 |
 |
| |
HIV-1 resistance in Mozambican infants following prophylaxis with single-dose nevirapine (sdNVP)
|
| |
| |
Ana Judith Blanco
University of Washington, Children's Hospital in Seattle, Health Alliance International, Mozambique Ministry of Health
The selection, transmission and persistence of drug-resistant HIV-1 in infants prophylaxed with single-dose nevirapine (sdNVP) varies by the timing of infection
Mark A Micek1,2, Ana Judith Blanco1, Ingrid A Beck3, Sandra Dross3, Eduardo Matediane4, Laurinda Mantunha1, Pablo Montoya1,2, Ilesh Jani4, Jennifer Kasper1, Kristy Siedel3, Soren Gantt2,3, Lilia Jamisse4, Stephen Gloyd1,2, Lisa M Frenkel*,2,3,
1Health Alliance International, Seattle, WA, USA, 2University of Washington, Seattle, WA, USA, 3Seattle Children's Hospital Research Institute, Seattle, WA, USA, 4Mozambique Ministry of Health, Mozambique, Africa
ABSTRACT: Infants infected with HIV-1 despite sdNVP may often harbor nevirapine-resistant viruses (rNVP-HIV-1). They hypothesized that the timing of nevirapine selective pressure relative to the time HIV-1 first infects and expands within infants effects the selection and persistence of rNVP-HIV-1. They are conducting a prospective observational cohort study of about 700 Mozambican infants following prophylaxis with sdNVP. The timing of HIV-1 infection was estimated using nested PCR for HIV-1 pol in dried blood spots collected at birth and every two weeks during the first 2 months of life, and then every 4-8 weeks until 12 months old. Concentrations of rNVP-HIV-1 were determined using quantitative PCR and quantitative oligonucleotide ligation assays for K103N, V106M, Y181C and G190A. All 24 infants infected in utero (defined as HIV-1 detected at birth) had wild-type viruses at birth. 19 of these had established in utero infection with a high and stable HIV-1 DNA load at birth, and 16 (84.2%) of these had selection of rNVP-HIV-1. rNVP-HIV-1 concentrations peaked between 2-8 weeks of age, and then regressed. In contrast, infants with acute peripartum infection (n=28), with either a small or undetectable viral population at birth that increased before 8 weeks of age, had lower rates of rNVP-HIV-1 (n=11/28; 39.3%). Mutant viruses comprised 100% of the viral populations in 7/11 (63.6%), which persisted at high concentrations during the first year of life. The selection, transmission and persistence of rNVP-HIV-1 in infants following sdNVP varies by the timing of their infection. Resistant viruses are generally selected when infection is well-established prior to birth. In contrast, acute HIV-1 infection during nevirapine pressure results in virus populations comprised of mixed, wild-type, or mutant genotypes, corresponding possibly to virus acquisition slightly prior, immediately prior, or after nevirapine dosing. rNVP-HIV-1 appears to persist longer when transmitted or selected during acute infection, implying a greater risk for failure of nevirapine-based ART. Our findings suggest that testing infants exposed to sdNVP for nevirapine resistance prior to ART could identify which infants may benefit from nevirapine- vs. non-nevirapine (i.e. protease inhibitor)-containing ART due to drug resistance.
Implications of Study
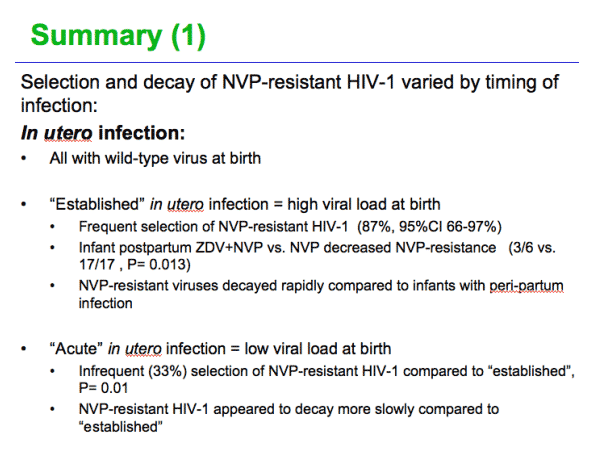
The rapid decay of NVP-resistant virus among the large group of infants with "established" in utero infection suggests that NVP-ART may be effective after an interval of time, as demonstrated for adult women (S Lockman et al. NEJM 2007)
The persistence of high concentrations of mutant virus following acquisition of 100% NVP-resistant HIV-1 suggests that NVP-based ART is unlikely to ever be efficacious in this small group of infants
The absence of NVP-resistant HIV-1 in the substantial number of infants with acute in utero or peripartum infection, and the rapid decay of mutants in infants with established in utero infections, suggests a role for drug-resistance testing prior to administering ART to infants given sdNVP
Addition of maternal +/- infant ZDV to sdNVP reduces transmission of HIV-1 and reduces the selection of NVP-resistance mutants, questioning the rationale of sdNVP-only programs

We hypothesized that the time at which an infant acquires HIV-1 relative to the selective pressure of sdNVP would determine the selection and longevity of the drug-resistant HIV-1 population within the infant. This is important the persistence of mutants will likely affect the outcome of NVP-ART. Understanding the dynamics of NVP-resistant viruses in infants has broad implications as most countries with a high prevalence of HIV-1 infection use sdNVP for pMTCT, and in these communities NVP-based ART is most economical choice for treatment of HIV-1 disease.
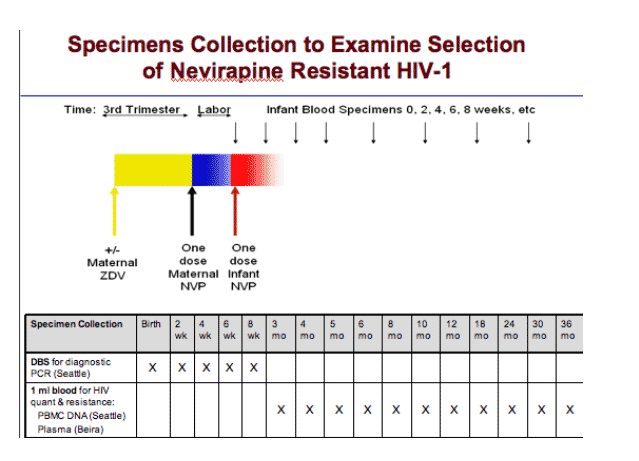
Newborns were recruited through their HIV-1 infected mothers at an HIV treatment facility prior to birth or from maternity wards at the time of delivery. Eligible infants included those who took a pMTCT regimen containing sdNVP.
To diagnose, determine the timing of HIV-1 infection, and quantify viral load, as well as the proportion of NVP-resistant virus in infants, whole blood was collected by heel-stick on FTAŽ filter paper cards (Whatman, Inc) at birth and at 2, 4, 6, and 8 weeks (w) of age, and by venipuncture at 3, 4, 5, 6, 8, 10, 12, 18, 24, 30 and 36 months (m) of age
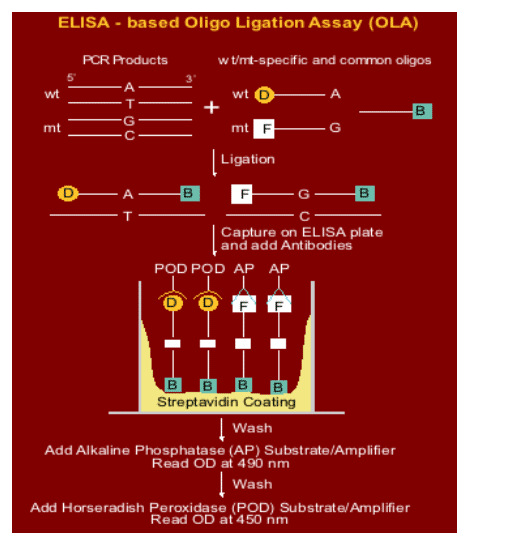
NVP-resistant HIV-1 mutants were detected and the size of each population estimated using an oligonucleotide ligation assay (OLA)
OLA reagents were adapted to detect four specific mutations in HIV-1 subtype C pol (K103N, V106M, Y181C, and G190A) conferring high-level resistance to NVP
A total of 100-300 copies of virus were evaluated from each specimen to ensure the ability to detect mutant viruses present at low concentrations. Specimens from all available study visits were evaluated in the same OLA plate to avoid inter-assay variability.
In the OLA, the PCR amplicon reacts with oligonucliotides specific for each mutant or wild-type genotype, with specificity conferred by the ligase enzyme. The ligated products are detected in an EIA reaction.
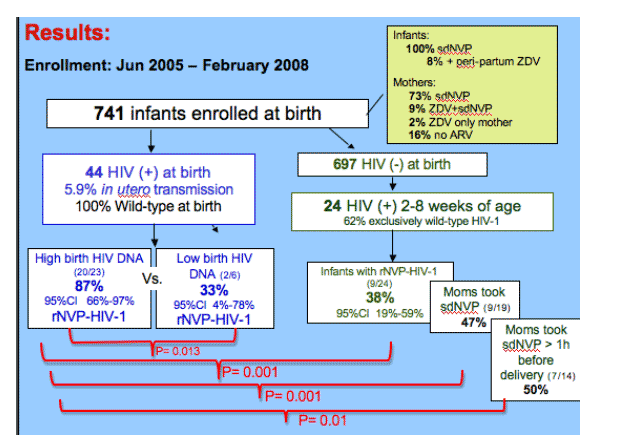
All 39 infants infected in utero had sufficient virus to assay 100 copies, had wt virus at birth. Selection of r-NVP-HIV-1 was greater in in utero infected infants with high versus low HIV-1 DNA loads at birth (n=87% vs. 33%.
Peripartum-infected infants had significantly less NVP-resistant-HIV-1 with an overall rate of 38%. A comparison of only the infants born to women who took sdNVP, also revealed a lower proportion of resistance in infants with peripartum compared to established in utero infection (9/19; 47%, 95%CI 24%-71% vs. 19/20; 95%, 95%CI 75%-100%; p=0.001). And this difference persisted when comparing infants whose mother's took sdNVP at more than 1 hour prior to delivery (7/14; 50%, 95%CI_23%-77% vs. 16/17; 94%, 95%CI 71%-100%; p=0.011).
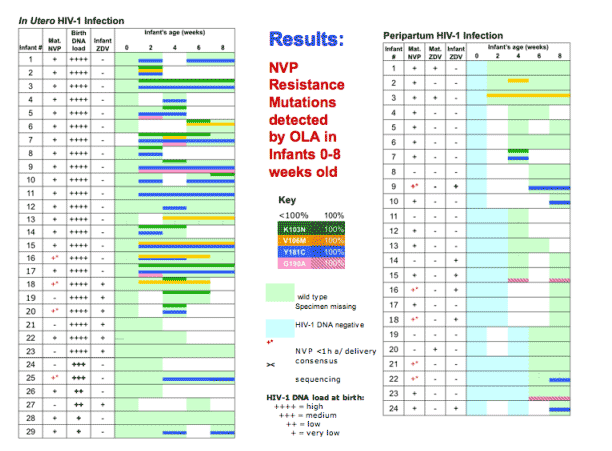
This figure shows the mutant codons selected for each infant infected in utero, with high and low viral loads at the time of birth, and in the peripartum period. Green indicated Wild-type virus, light blue indicates No HIV amplified, white indicates missing specimens, and bold colors mutants, and colors with slashes indicated 100% mutant.
Among infants with established in utero infection all had wild-type virus at birth. One or more mutant codons were selected in all that took sdNVP without ZDV, and was significantly less (p=0.013) frequent, occurring in only half that took both. The mutants peaked at 2-8 weeks and then regressed.
Infants will (with?) acute in utero had resistance less frequently but when detected it was high-level, peaking at 100% resistance.
Peripartum-infected infants also had NVP-resistant-HIV-1 less often that established in utero. However, most of those with resistant virus had 100% mutant from the time of the first (+) specimen, suggesting acquisition of NVP-resistant virus.
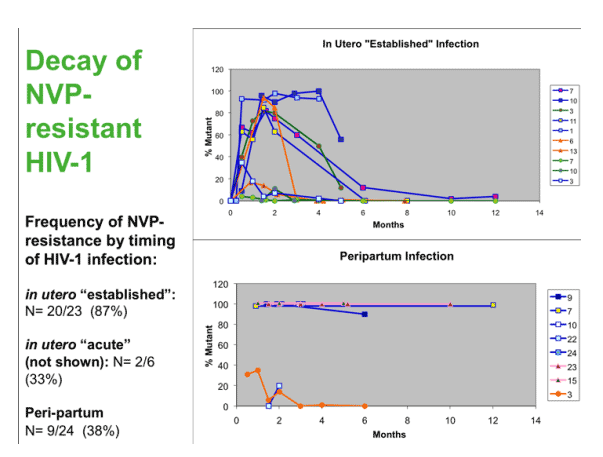
The selection and persistence of NVP-resistant HIV-1 in infants following sdNVP is variable, and appears to be influenced by the size of the virus population at the onset of NVP selective pressure: Nearly all In utero infected infants with large virus populations, here referred to as "established", experience transient selection of rNVP-HIV-1, which decays rapidly. Those with in utero infection but small virus populations at birth mostly appear to have insufficient viral diversity and replication to generate NVP-resistant-HIV-1 - thus, most remain with wild-type virus. Among infants with peripartum infection, NVP-resistant HIV-1, most have exclusively wild-type virus, although a significant minority appear to acquire NVP-resistant virus, and a few acquire a mixture of drug-resistant and sensitive genotypes. Transmitted mutants appear to persist longer than selected mutants, probably because these mutants populate the viral reservoirs.
The selection and persistence of NVP-resistant HIV-1 in infants following sdNVP is variable, and depends on the size of the virus population at the onset of NVP selective pressure: The In utero infected infants with large virus populations experience transient selection of rNVP-HIV-1, which decays rapidly. Those with small virus populations may have insufficient viral diversity and replication to select NVP-resistant-HIV-1.
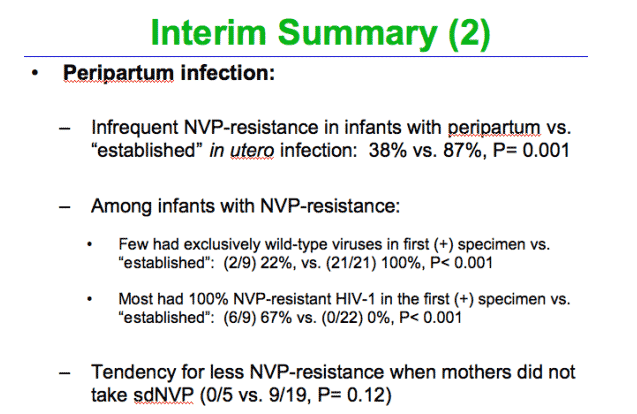
Most babies acquiring HIV in the peripartum period had wild-type virus, with only 38% having resistance. NVP-resistant HIV-1 can also be transmitted in the peripartum period. Our study may have underestimated NVP resistance as 5 of the 24 mothers did not take NVP, and none of their infants had resistant viruses detected. Transmitted mutants persist longer than selected mutants, as the mutants likely populates the long-lived viral reservoirs.
|
| |
|
 |
 |
|
|