 |
 |
 |
| |
SVR Improves Survival, Risk for Liver Cancer, Decompensated Liver Disease and Liver Transplant/Death - Also, Transient Viral Suppression (breakthroughs/relapsers) Improves Clinical Outcomes Too
|
| |
| |
Reported by Jules Levin
AASLD Oct 31-Nov 3 209, Boston, MA
The main outcome of this study is that SVR reduces risk for HCC, decompensation, liver transplant/liver death any other clinical outcomes compared in the HALT-C Study, which looked at peg/rbv nonresponders and included patients with advanced disease. This is good because some federal government committees have suggested there is not adequate proof that SVR improves clinical outcomes for patients and therefore have not supported federal funding for programs for patients including HCV screening and surveillance and therapy as well.
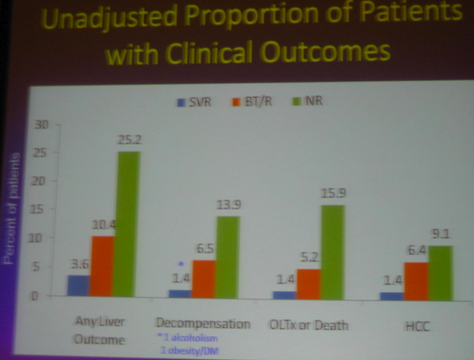
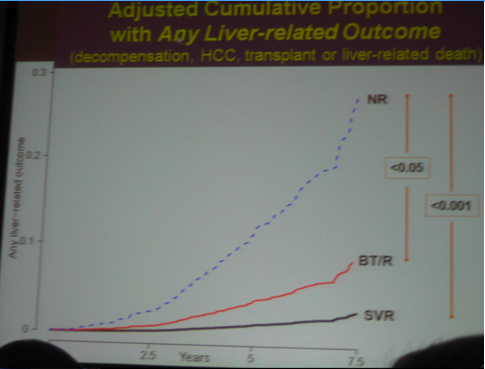
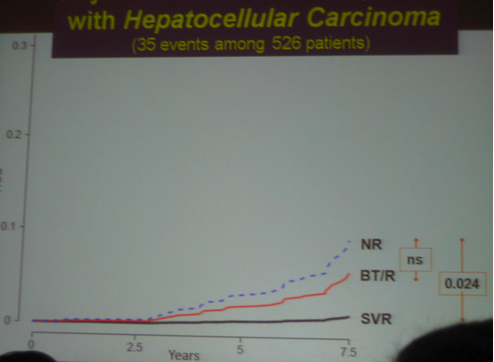
Although the main outcome of this study was that that SVR reduces cancer, death, clinical outcomes, decompensation.....However......As we know patients included in the HALT-C study had advanced liver disease. Two years ago the HALT-C investigators reported at AASLD that maintenance therapy did NOT provide clinical benefit. These headlines made quite a splash and many clinicians stopped using maintenance therapy as patients & clinicians presumed it had no benefit. Several months later at EASL in April HALT-C investigators presented that if patients had a 4-log viral load reduction there appeared to be some benefit. Now at this AASLD last week the same investigators reported patients in HALT-C who were breakthroughs/relapsers, "patients with viral suppression", had significantly reduced risk of developing any liver-related complication when compared with non-responders; see graphs and table below: reduced risk for "any outcome, decompensation liver transplant/liver death" appear statistically significant, reduced risk for HCC was not statistically significant between nonresponder and breakthrough/relapser but there appeared to be a reduced risk in the number of events.
By the way the HALT-C researchers have refused to share slides after their presentations so they can publish their results. They refused to share the slides 2 years ago after they presented at AASLD that maintenance therapy was NOT beneficial. If the original design of their study inquiries had been better designed to include looking at partial responders we would have had better results 2 years ago and better understood how to use maintenance therapy.
AUTHOR SUMMARY
When compared with non-responders patients with SVR had a reduced risk of
-- Decompensated liver disease
-- HCC
-- Liver transplant/liver-related death
Patients with transient viral suppression (i.e., viral breakthrough/relapse) had:
-- a risk of clinical outcomes intermediate between non-responders and patients with SVR and
-- a significantly reduced risk of developing "any liver-related complication" when compared with non-responders.
-- HCC occurred up to 6 years following SVR, although the risk was significantly reduced
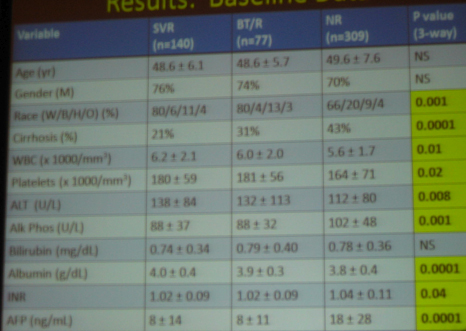
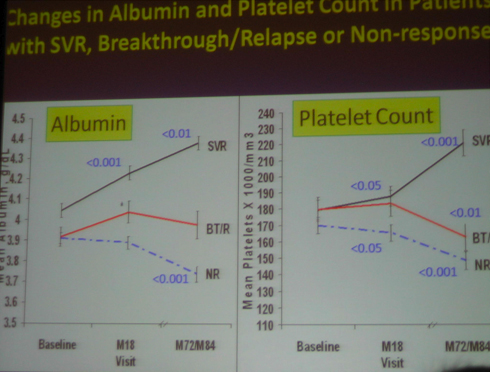
Platelet count and albumin improved after SVR. Conversely, platelet count and albumin -- worsened in non-responders and in breakthrough/relapsers
AUTHORS CONCLUDED
-- HCV patients with advanced liver disease who achieved an SVR had an 80-90% reduction in liver-related clinical outcomes.
-- Patients with transient suppression of HCV RNA had a 50% reduction in liver-related clinical outcomes.
-- Blood tests associated with liver function improved after achieving an SVR suggesting that improved liver function improves in patients with advanced liver fibrosis who achieve an SVR
AIMS
To determine the effect of SVR on:
-- the development of decompensated liver disease, HCC, liver transplant or liver-related death.
To determine the effect of transient suppression of HCV/RNA on the risk of developing liver-related clinical outcomes
HALT-C Inclusion/Exclusion Criteria
Inclusion
-- non-response to interferon +/- ribavirin
Exclusion
-- other liver diseases, or HIV
-- history of decompensated liver diseases or HCC
-- Child-Turcotte-Pugh score >/-7
DESIGN OF CURRENT STUDY
BT/R = breakthrough/relapser
NR = nonresponder

PRIMARY OUTCOMES
Liver-Related clinical outcomes (through 7.5 yrs)
1. Any liver-related outcome (decompensation, HCC, OLTx, liver-related
2. Decompensated liver disease: ascites, variceal bleeding, SBP or encepthalopathy
3. hepatocellular carcinoma, (liver cancer)
4. Liver transplant or liver-related death
Blood tests: CBC, liver panel, INR
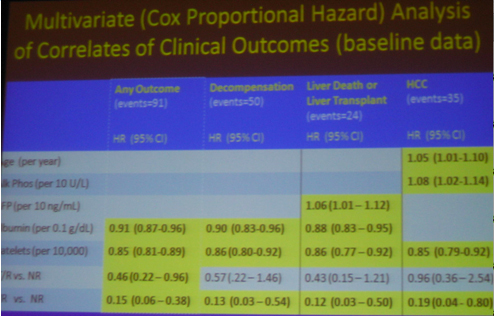
Variables not significant in any model:
- Cirrhosis vs fibrosis
- Race, and
- AST/ALT ratio
|
| |
|
 |
 |
|
|