 |
 |
 |
| |
|
Review of HIV+ Patient's Immunological Response Rate to High-dose HBV Re-vaccination Series: Cd4 & viral load predict response
|
| |
| |
Reported by Jules Levin CROI 2009 Feb 8-12
Wilson Ly*, D Nguyen, T Phengrasamy, and S Follansbee
Kaiser Permanente Med Care Prgm, San Francisco, CA, US
AUTHOR CONCLUSION
The preliminary data of this study suggest that more patients are likely to respond to the conventional HBV vaccine dose series if their CD4 counts are above 350 or they have undetectable viral load at the time of vaccination. In patients not responding to the conventional dose series, re-vaccinating with the high dose series may be a promising option. Future studies may include the comparison of high dose with conventional dose re-vaccination in HIV+ patients not responding to conventional dose vaccination series.
AUTHOR DISCUSSION
The immunological response rate (51.4%) of KPSF HIV positive patients to conventional dose HBV vaccination series is consistent with the 16 - 70% range reported in the literature. Our findings further support the observation of the poor outcome with the standard HBV vaccine doses in HIV positive patients.
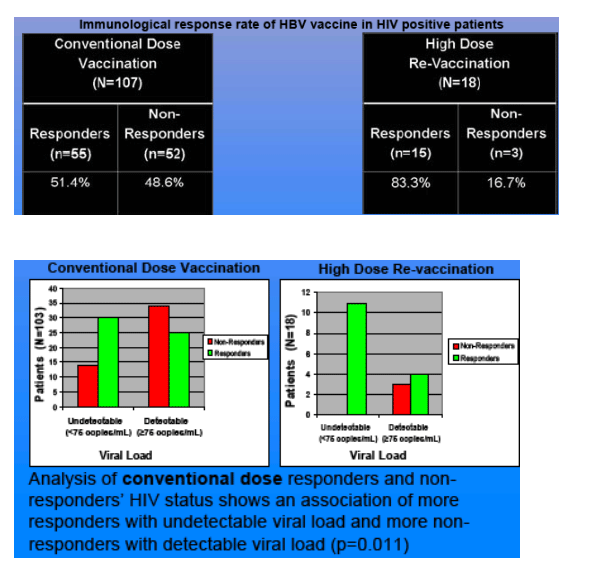
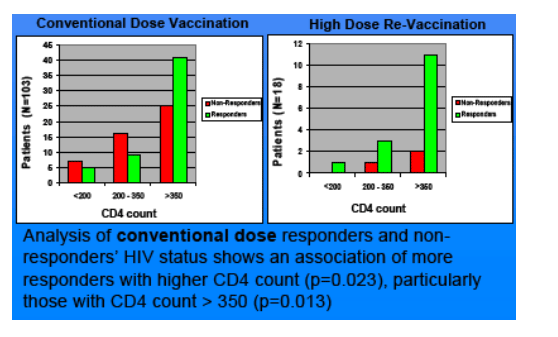
Our results suggest that patients with undetectable viral load or higher CD4 count, particularly > 350, are more likely to respond to the conventional dose series. Thus, it may be beneficial to vaccinate patients with conventional dose series if their CD4 counts are still above 350 and HIV viral load is undetectable. Whether response rate is independently associated with HIV viral load or CD4 count has not been evaluated. The preliminary data of this study suggests that more patients are likely to respond to the conventional HBV vaccine dose series if their CD4 counts are above 350 or they have undetectable viral load at the time of vaccination. In patients not responding to the conventional dose series, re-vaccinating with the high dose series may be a promising option.
For patients with CD4 <350 or detectable viral load, HBV vaccination is still necessary but follow-up serology testing 1-2 months after the last dose should be conducted order to identify the non-responders to the conventional dose series. A second HBV vaccination series may then be considered, if a patient is found to have anti-HBs level < 10 IU/L.
Our patients' immunological response rate to high dose HBV re-vaccination series is 83..3% but it has not reached statistical significance due to small sample size. Additional sub-analysis would be useful to determine if 83.3% response rate may be secondary to the effect of re-vaccination rather than a result of using a higher dose of the vaccine
Background: The Centers for Disease Control and Prevention (CDC), US Public Health Service, and the Infectious Disease Society of America recommend routine hepatitis B virus (HBV) screening and immunization for all HIV+ patients to prevent HBV infection and related complications. The effort to induce HBV immunity in HIV patients has been poor with standard HBV vaccine doses. Several studies have demonstrated increased vaccination success when high dose HBV vaccines were administered to HIV patients during the initial vaccination and limited data for the re-vaccination attempts. This study is intended to describe the immunological response rate (hepatitis B surface antibody [anti-HBs] ≥10 IU/L) to re-vaccination with high dose HBV vaccine in adult HIV+ patients at Kaiser Permanente San Francisco (KPSF) Medical Center. Response rate to conventional dose HBV vaccination series is included for comparative reference.
Methods: Retrospective chart review of all adults HIV+ patients at KPSF Medical Center who received conventional and high-dose HBV vaccine with completed follow-up serology testing between January 2007 and March 2008. Primary endpoint is the response rates to standard and high-dose HBV vaccination. Secondary endpoints are the association of HIV viral load and CD4 count trend in responders and non-responders to HBV vaccination.. Statistical significance of the endpoints is analyzed using c2 tests, and p <0.05 is considered as significant.
Results: Of the 107 who completed the conventional doses HBV vaccination series, 55 were responders (51.4%) and 52 non-responders (48.6%); 12 non-responders to the conventional dose series completed the high-dose re-vaccination series, with 11 responders (92%) and 1 non-responder (8%). Analysis of the conventional dose responders and non-responders HIV status shows a trend of more responders with higher CD4 count (p = 0.023), particularly those with CD4 count >350 (p = 0.013). Conventional dose responders and non-responder's HIV status shows a trend of more responders with undetectable viral load and more non-responders with detectable viral load (p = 0.0105).
Conclusions: The preliminary data of this study suggest that more patients are likely to response to the conventional HBV vaccine dose series if their CD4 counts are >350 and they have undetectable viral load at the time of vaccination. In patients not responding to conventional dose series, re-vaccinate with high dose series may be a promising option.
INTRODUCTION
éThe CDC, U.S. Public Health Service and the Infectious Disease Society of America recommend routine hepatitis B virus (HBV) screening and immunization for all HIV positive patients to prevent HBV infection and related complications
éAdult HBV vaccine formulations currently available in the U.S.
Conventional dose
- Engerix-B: 20 mcg/ml
- Recombivax-HB: 10 mcg/ml
- Twinrix (Hepatitis A & B)
High dose (FDA: for dialysis patients)Recombivax-HB: 40 mcg/ml
- Vaccination schedule1 ml injection IM x 3
- 0, 1-2, 6-12 months
Follow up serology testing
- Consider checking anti-HBs 1-2 months after the last injection
- Responders: anti-HBs ≥ 10 IU/L
Response rate (literature)
- Healthy patients: >90%
- HIV+ patients: 17 - 60%
Emerging data suggest possible improved response rate with high dose (40 mcg/ml) vaccination & re-vaccination
At Kaiser Permanente San Francisco HIV Program, some providers consider using high dose (40 mcg/ml) re-vaccination series in non-responders to conventional series. Schedule for re-vaccination is at 0,1, 2 months.
RESULTS
|
| |
|
 |
 |
|
|