 |
 |
 |
| |
Entecavir Maintains a High Genetic Barrier to HBV Resistance Through 6 Years in Naïve Patients
|
| |
| |
Digestive Disease Week 2009, McCormick Place, Chicago Illinois, May 30-June 4, 2009
Reported by Jules Levin
D.J. Tenney1, K.A. Pokorowski1, R.E. Rose1, C.J. Baldick1, B.J. Eggers1, J. Fang1, M.J. Wichroski1, U. Diva1, D. Xu1, R.B. Wilber1, H. Brett-Smith1 & U.H. Iloeje1
1Research and Development, Bristol-Myers Squibb Company, United States
Summary of Results
Nucleoside-Naïve:
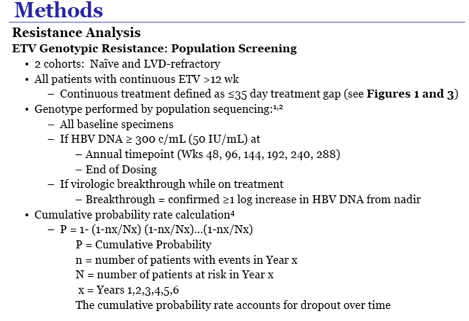
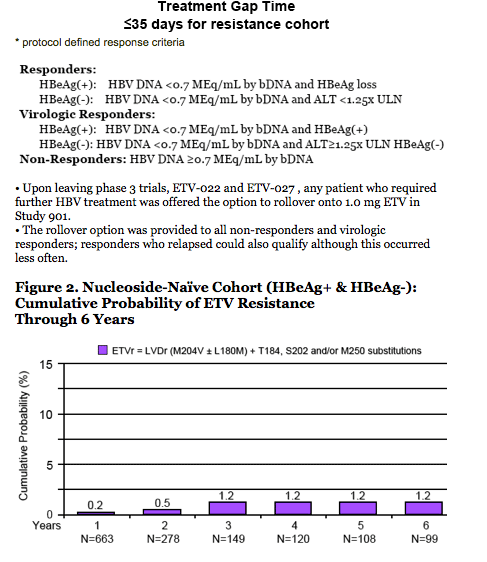
· Resistance is rare through 6 years
- 1.2% cumulative probability of genotypic resistance.
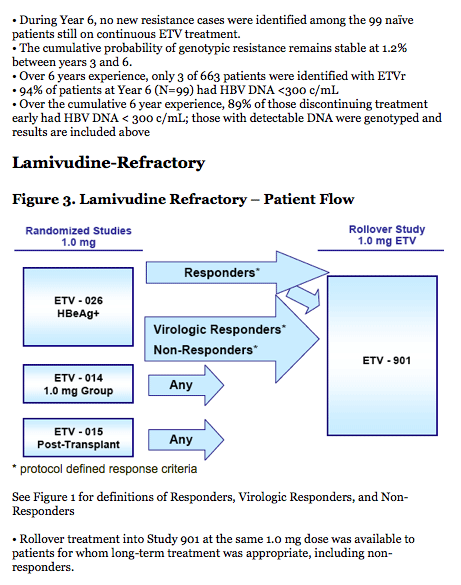
LVD-Refractory:
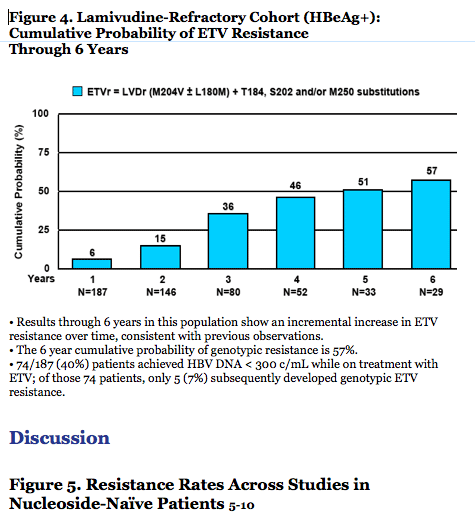
· The 6 year cumulative probability of genotypic resistance is 57%.
- 74/187 (40%) patients achieved HBV DNA < 300 c/mL while on treatment with ETV; of those
74 patients, only 5 (7%) subsequently developed genotypic ETV resistance.
Conclusion
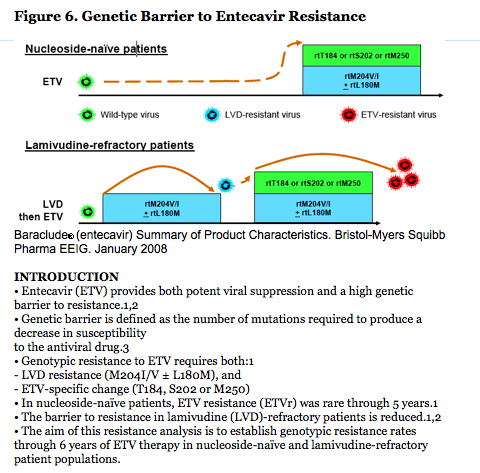
· ETV has high potency and high genetic barrier to resistance in nucleoside-naïve patients (Figure 6).
· In lamivudine-refractory patients, ETV potency and genetic barrier are reduced and an incremental increase in resistance is demonstrated over time (Figure 6).
- Favorable prognostic subgroups can be identified by early response to treatment.
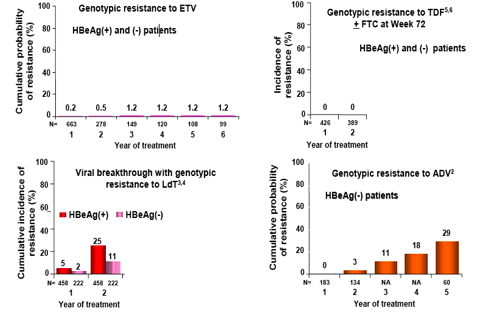
· No two drugs have data that can be directly compared to each other as they are limited by differences in study populations and analysis methodologies.
· The above figure shows that ETV has a favorable resistance profile in naïve patients over a period of multiple years.
Limitations
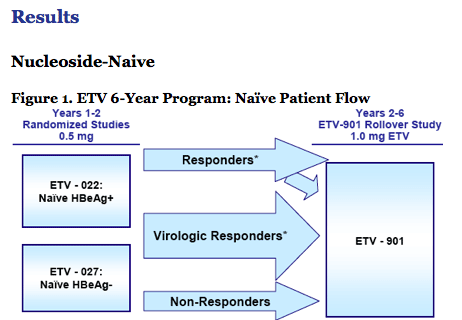
· For naïve patients, the rollover results in treatment with a higher ETV dose in Study 901.
· The rollover process may have facilitated patient drop-out.
· Study design favored early discontinuation of those who were successfully treated based on patient management endpoints.
REFERENCES
1. Tenney DJ, et al. Hepatology 2009;49(5):1503-1514.
2. Colonno RJ, et al. Hepatology 2006;44:1656-1665.
3. Lok AS, et al. Hepatology 2007;46:254-265.
4. Pawlotsky JM, et al. Gastroenterology 2008;134:410-411.
5. Chang TT, et al. J Gastroenterol Hepatol 2004;19:1276-1282.
6. Hadziyannis S, et al. Gastroenterology 2006;131:1743-1751.
7. Lai CL, et al. N Engl J Med, 2007;357:2576-2578.
8. Liaw YF, et al. Gastroenterology 2009;136:486-495.
9. Marcellin P, et al. N Engl J Med 2008;359:2442-2455.
10. Snow-Lampart A, et al. Hepatology 2008;48(suppl.): 745A.
|
| |
|
 |
 |
|
|