 |
 |
 |
| |
Entecavir Maintains a High Genetic Barrier to HBV Resistance Through 6 Years in Naive Patients
|
| |
| |
EASL April 23-26 2009 Copenhagen Denmark
Reported by Jules Levin, NATAP
D.J. Tenney1, K.A. Pokorowski1, R.E. Rose1,
C.J. Baldick1, B.J. Eggers1, J. Fang1, M.J. Wichroski1,
U. Diva1, D. Xu1, R.B. Wilber1, H. Brett-Smith1 & U.H. Iloeje1
1Research and Development, Bristol-Myers Squibb Company, United States
Oral Presentations
Session Title: Parallel Session 2: HEPATITIS B VIRUS THERAPY
Presentation Date: Apr 23, 2009
ENTECAVIR MAINTAINS A HIGH GENETIC BARRIER TO HBV RESISTANCE THROUGH 6 YEARS IN NAIVE PATIENTS
D.J. Tenney, K.A. Pokornowski, R.E. Rose, C.J. Baldick, B.J. Eggers, J. Fang, M.J. Wichroski, U.A. Diva, D. Xu, R.B. Wilber, H. Brett-Smith, U.H. Iloeje
Research and Development, Bristol-Myers Squibb Company, Wallingford, CT, USA
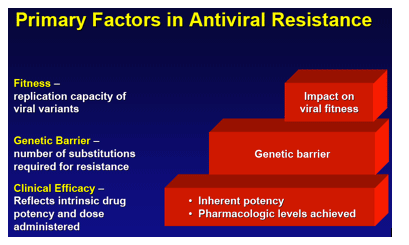
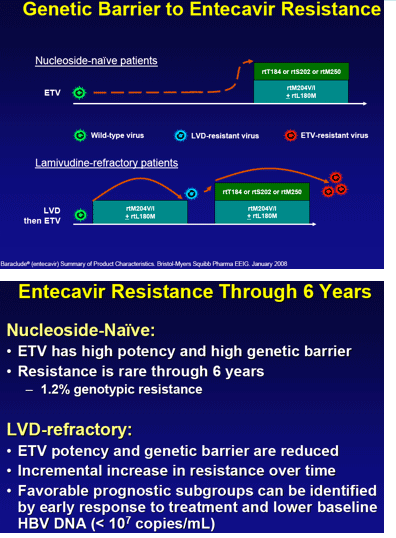
Background/ aims: Entecavir (ETV) provides both potent viral suppression and a high genetic barrier to resistance. As a result, in nucleoside-naive patients, ETV resistance (ETVr) was rare through 5 years. The barrier to resistance in lamivudine (LVD)-refractory patients is reduced.
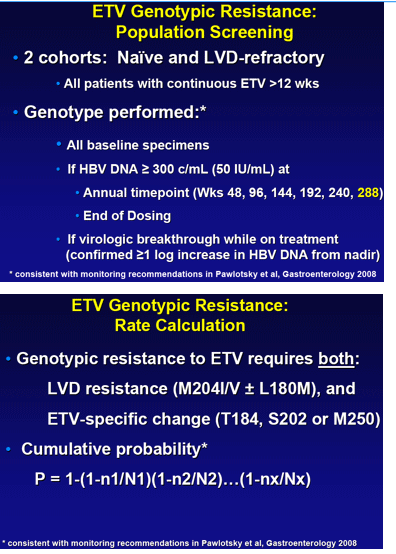
Methods: All patients receiving continuous therapy in registrational trials were monitored for resistance through year 6. Sequencing was performed on serum samples with detectable HBV DNA (≥300 copies/mL) at each cross-sectional end-of-year analysis, or with viral breakthrough at anytime, or at discontinuation from study with detectable HBV DNA. Cumulative probabilities of resistance were determined through year 6.
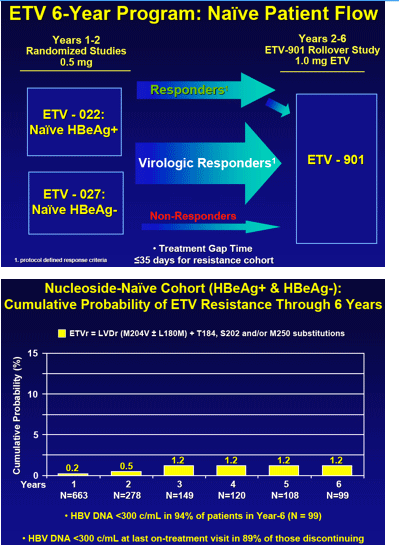
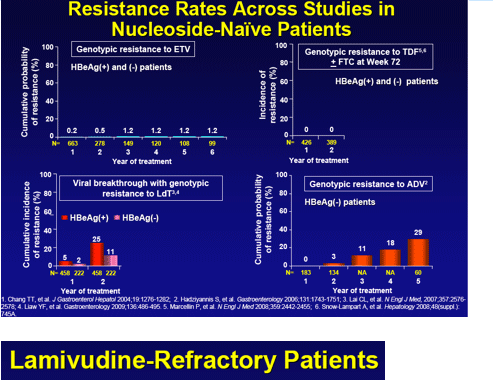
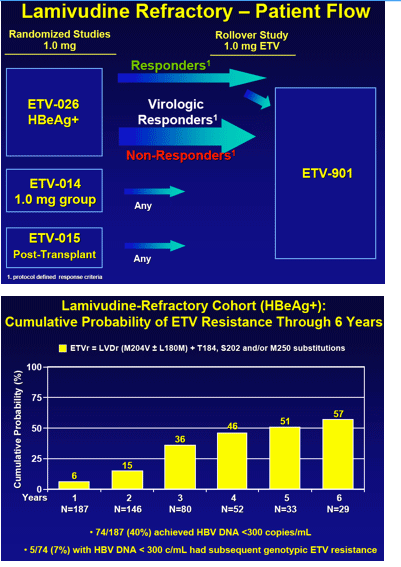
Results: In years 1 through 6, respectively, 663, 278, 149, 120, 108 and 99 nucleoside-naive patients were treated and monitored, with 94% in year 6 having HBV DNA < 300 copies/mL. No patient in year 6 showed emerging ETVr at T184, S202 or M250 ± LVD resistance (LVDr) M204I/V±L180M. The cumulative probability of genotypic ETVr in nucleoside-naive patients remained at 1.2% through 6 years. Among LVD-refractory patients treated with ETV, 187, 146, 80, 52, 33 and 29 were monitored in years 1 through 6, respectively. The cumulative probabilities of genotypic ETVr at years 1 through 6 were 6%, 15%, 36%, 47%, 51%, and 57% respectively, and of virological breakthrough with ETVr was 50% through year 6. Among the 74 LVD-refractory patients who achieved undetectable HBV DNA on ETV, 5 subsequently developed ETVr.
Conclusions: ETVr remains rare (1.2%) in nucleoside-naive patients through 6 years. LVDr HBV has a reduced resistance barrier to ETV, and patients with LVDr HBV may benefit from add-on or combination therapy.
|
| |
|
 |
 |
|
|