| |
Glucocorticoid-induced osteoporosis: hope on the HORIZON-COMMENT once yearly infusion therapy better than once weekly
|
| |
| |
The Lancet, Volume 373, Issue 9671, Pages 1225 - 1226, 11 April 2009
doi:10.1016/S0140-6736(09)60704-2
Luigi Gennari aEmail Address, John P Bilezikian b
Department of Internal Medicine, Endocrine Metabolic Sciences and Biochemistry, University of Siena, Siena 53100, Italy
b Metabolic Bone Diseases Unit, Division of Endocrinology, Department of Medicine, College of Physicians and Surgeons, Columbia University, New York, NY, USA
Glucocorticoids are used to treat many disorders. Their negative effect on skeletal health is a substantial drawback, such that their use is the most frequent secondary cause of osteoporosis.1 30-50% of patients who take glucocorticoids chronically have a fracture. Glucocorticoids decrease bone formation by reducing the lifespan of osteoblasts and osteocytes (figure).
Figure
Effects of glucocorticoids, bisphosphonates, and teriparatide on bone cells
Dotted lines indicate potential effects of bisphosphonates.
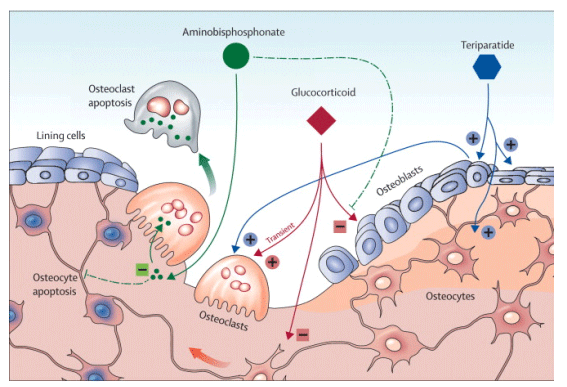
Prevention and treatment of glucocorticoid-induced osteoporosis should be a major consideration for people who need these drugs chronically. Sufficient amounts of calcium and vitamin D are mainstays of the therapeutic approach, along with bisphosphonates.2 Bisphosphonates reduce bone resorption through their actions on osteoclasts (figure). Oral alendronate and risedronate are effective for treatment of glucocorticoid-induced osteoporosis, and risedronate can also prevent this complication.3, 4
Despite these indications, patients do not always adhere to oral regimens of bisphosphonates. Adherence is increasingly recognised as important for therapeutic effectiveness.5 Intravenous bisphosphonates that can be used intermittently with the same or greater antiresorptive potency than are the oral drugs present potential advantages for treatment of glucocorticoid-induced osteoporosis.
In The Lancet today, David Reid and colleagues6 report the results of a 1-year multicentre randomised trial (HORIZON) to ascertain non-inferiority of an intravenous infusion of zoledronic acid (5 mg, given once a year) compared with an oral dose of risedronate (5 mg a day) for prevention and treatment of glucocorticoid-induced osteoporosis. Not only was zoledronic acid non-inferior to risedronate in increasing bone mineral density of the lumbar spine but it was also superior in terms of a significantly greater percentage change in bone mineral density in this site. Superiority was also found for bone mineral density for the femoral neck, trochanter, and total hip, and in reduction of markers of bone turnover. These differences were evident within 6 months of treatment. The size of the effects of zoledronic acid on yearly changes in bone mineral density was similar to data of pivotal clinical trials that included a placebo group.7
During the first 3 days after the start of treatment, zoledronic acid was associated with more adverse events (acute-phase reaction) than risedronate, with influenza-like symptoms and pyrexia. Thereafter the tolerability and safety profile of the two bisphosphonates was similar. Because study participants were not selected on the basis of low bone mass, these results are applicable to a wide range of individuals who are candidates for prevention or treatment of glucocorticoid-induced osteoporosis.
The study by Reid and colleagues had no placebo arm, was of short duration, and was not powered to see any differences in fracture incidence. The inference that the greater effects of zoledronic acid than with risedronate on bone mineral density and bone turnover could be extrapolated to actual differences in antifracture efficacy requires a cautionary note. Bone mineral density and bone turnover account only incompletely for fracture efficacy. Moreover, even though bone mineral density is an important predictor of fracture risk in glucocorticoid-induced osteoporosis, fractures often happen in patients without a consistent reduction in bone mineral density, possibly due to the effects of glucocorticoids on other components of bone strength.1 Studies are therefore needed to address the issue of fracture reduction directly in a comparative trial of zoledronic acid and other bisphosphonates.
Although a once-yearly intravenous infusion of zoledronic acid would seem to have obvious advantages over an oral regimen, we still do not know the best dosing strategy for zoledronic acid in terms of cost-effectiveness and safety of long-term regimens in glucocorticoid-induced osteoporosis.. This information is especially important in view of the long duration of action of zoledronic acid and concerns about possible deleterious effects from long-term oversuppression of bone turnover.8
Studies are also needed to assess the effects of zoledronic acid on other indicators of bone quality in glucocorticoid-induced osteoporosis. Importantly, different experimental observations show that this disorder is caused largely by inhibition of bone formation and not by augmented bone resorption.
Glucocorticoid-induced osteoporosis is thus different from postmenopausal osteoporosis.9 Indeed, bone resorption increases early and only transiently in glucocorticoid-induced osteoporosis. Thus are inhibitors of bone resorption such as bisphosphonates the ideal treatment for glucocorticoid-induced osteoporosis?
Anabolic drugs such as teriparatide, which stimulate bone formation by acting on osteoblasts and osteocytes (figure), could lead to a more compelling rationale for use than bisphosphonates. This drug is superior to alendronate in glucocorticoid-induced osteoporosis not only for changes in bone mineral density but also in terms of morphometric vertebral fractures.10 On the other hand, bisphosphonates can prevent osteoblast and osteocyte apoptosis in vivo and in vitro,11, 12 raising the possibility that perhaps part of their antifracture efficacy in glucocorticoid-induced osteoporosis might result from preservation of the integrity of the osteocyte network and extension of the working life of bone-forming cells. Further studies are needed to address these issues, so that we have greater assurance about the effectiveness of available antiresorptive and anabolic drugs for prevention and treatment of glucocorticoid-induced osteoporosis.
LG declares that he has no conflicts of interest. JPB has received honoraria for invited talks and consultancies from Merck, Procter & Gamble, Sanofi-Aventis, Lilly, Novartis, GlaxoSmithKline, and NPS Pharmaceuticals, and has received research funding or funding for equipment or drugs from Procter & Gamble, NPS Pharmaceuticals, and GlaxoSmithKline.
|
|
| |
| |
|
|
|