| |
Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Diseases
|
| |
| |
STATE-OF-THE-ART PAPER
J Am Coll Cardiol, 2009; 54:585-594, doi:10.1016/j.jacc.2009.02.084
Carl J. Lavie, MD*,*, Richard V. Milani, MD*,Mandeep R. Mehra, MD andHector O. Ventura, MD*
Department of Cardiovascular Diseases, Ochsner Medical Center, New Orleans, Louisiana
Division of Cardiovascular Diseases, University of Maryland School of Medicine, Baltimore, Maryland
Manuscript received February 5, 2009; accepted February 25, 2009.
Reprint requests and correspondence: Dr. Carl J. Lavie, Cardiac Rehabilitation, Ochsner Medical Center, 1514 Jefferson Highway, New Orleans, Louisiana 70121-2483 (Email: clavie@ochsner.org).
Abstract
Omega-3 polyunsaturated fatty acid (-3 PUFA) therapy continues to show great promise in primary and, particularly in secondary prevention of cardiovascular (CV) diseases. The most compelling evidence for CV benefits of -3 PUFA comes from 4 controlled trials of nearly 40,000 participants randomized to receive eicosapentaenoic acid (EPA) with or without docosahexaenoic acid (DHA) in studies of patients in primary prevention, after myocardial infarction, and most recently, with heart failure (HF). We discuss the evidence from retrospective epidemiologic studies and from large randomizedcontrolled trials showing the benefits of -3 PUFA, specifically EPA and DHA, in primary and secondary CV prevention and provide insight into potential mechanisms of these observed benefits. The target EPA + DHA consumption should be at least 500 mg/day for individuals without underlying overt CV disease and at least 800 to 1,000 mg/day for individuals with known coronary heart disease and HF. Further studies are needed to determine optimal dosing and the relative ratio of DHA and EPA -3 PUFA that provides maximal cardioprotection in those at risk of CV disease as well in the treatment of atherosclerotic, arrhythmic, and primarymyocardial disorders.
Fish oil is a whale of a story, that not surprisingly gets bigger with every telling.
-Rogans (1)
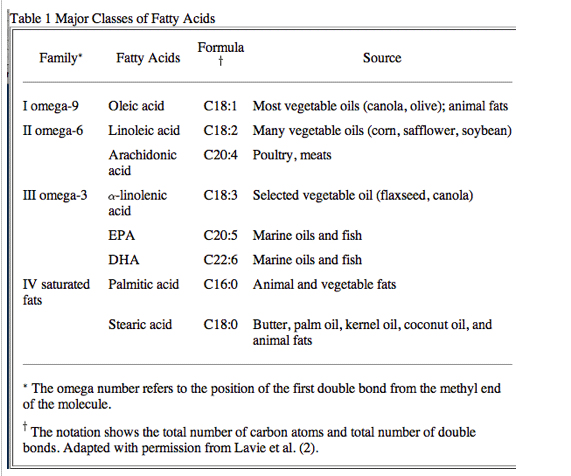
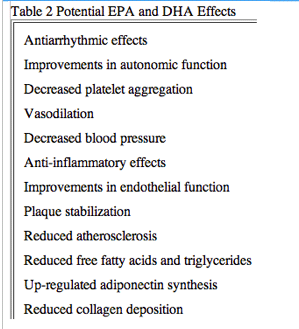
Fish oil is obtained in the human diet by eating oily fish, such as herring, mackerel, salmon, albacore tuna, and sardines,or by consuming fish oil supplements or cod liver oil. However, fish do not naturally produce these oils, but obtain them through the ocean food chain from the marine microorganisms that are the original source of the omega-3 polyunsaturated fatty acids (-3 PUFA) found in fish oils. Numerous prospective and retrospective trials from many countries, including the U.S., have shown that moderate fish oil consumption decreases the risk of major cardiovascular(CV) events, such as myocardial infarction (MI), sudden cardiac death (SCD), coronary heart disease (CHD), atrial fibrillation (AF), and most recently, death in patients with heart failure (HF) (2-8). Considerable attention has been directed at the various classes of fatty acids and their impact on the prevention and treatment of CV diseases (2) (Table 1). Most of the evidence for benefits of the -3 PUFA has been obtained for eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the long-chain fatty acids in this family. There is, however, some epidemiological support for a benefit from alpha-linolenic acid (ALA), the plant-based precursor of EPA. The American Heart Association (AHA) has currently endorsed the use of -3 PUFA at a dose of approximately 1 g/day of combined DHA and EPA, either in the form of fatty fish or fish oil supplements (in capsules or liquid form) in patients with documented CHD (9). The health benefits of these long-chain fatty acids are numerous and remain an active area of research (Table 2).
Background Epidemiologic Evidence
During the past 3 decades, numerous epidemiologic and observational studies have been published on the CV benefits of -3 PUFA (2-5). As early as 1944, Sinclair (10) described the rarity of CHD in Greenland Eskimos, who consumed a diet high in whale, seal, and fish. More than 30 years ago, Bang and Dyberg (11-13) reported that despite a diet low in fruit, vegetables, and complexcarbohydrates but high in saturated fat and cholesterol, serum cholesterol and triglycerides were lower in Greenland Inuit than in age-matched residents of Denmark, and the risk of MI was markedly lower in the Greenland population compared with the Danes. These initial observations raised speculation on the potential benefits of -3 PUFA (particularly EPA and DHA) as the protective "Eskimo factor" (14). Although a detailed review of all epidemiologic studies is beyond the scope of this article, data from Japan, Norway, Holland, and the U.S. have extended the seminal work of Bang and Dyberg (2,3,14). Recent evidence, however, has raised the concern that intrusion of Western dietary habits, including massive amounts of shortening and other saturated fats, into societies such as the Alaskan Native and Japanese may partly overwhelm the cardioprotective effects of -3 PUFA (14).
Trials in CHD
Harris et al. (15) have reviewed 25 trials that evaluated the risk of CHD events as a function of in vivo levels of -3 PUFA and showed that reduction in major CV events correlated inversely with the tissue levels of EPA, and even more so, with DHA.
Three large randomized trials have documented the effects of -3 PUFA in primary and especially in secondary prevention of CHD. In a randomized trial (DART -Diet and Reinfarction Trial]) (16) performed 2 decades ago in 2,033 men with recent MI, -3 PUFA, either in the form of oily fish or fish oil capsules,reduced 2-year all-cause mortality by 29% with the benefit almost entirely attributable to a reduction in CHD mortality. The reduction in CV events was particularly impressive in the subgroup who consumed fish oil capsules as opposed to simply increasing dietary fish consumption, likely indicating a threshold effect of -3 PUFA.
More recently, 2 major randomized control trials were performed. The GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell' Infarto Miocardico)-Prevenzione study (6) randomized 11,323 post-MI patients to -3 PUFA (1 capsule per day providing 850 mg of EPA/DHA in a 1.2:1 ratio; currently available as Lovaza, GlaxoSmithKline, Research Triangle Park, North Carolina) versus usual care (6,17,18). At the end of 1 year of follow-up, patients taking the fish oil supplement had a 15% reduction in the primary end point, including 21% and 30% reductions in total and CV mortality, respectively (Fig. 1). Further analyses showed that this endpoint reduction was driven by a highly significant 45%reduction in SCD, which was evident after only 4 months. In a subgroup analysis from this trial, the magnitude of reduction in total mortality and from SCD increased with progressive worsening of left ventricular (LV) systolic function (17). Long-term follow-up has continued to show reductions in major clinical events at 3.5-year follow-up (18).
In another trial, the JELIS (Japan EPA Lipid Intervention Study) trial (7), 18,645 patients (14,981 in primary prevention and 3,664 in secondary prevention) with hypercholesterolemia (70% women) were randomized to statin alone or statin and highly purified EPA 1,800 mg/day. At the end of the 5-year study, those randomized to EPA had a 19% reduction in major CV events (Fig. 2). Unlike the GISSI-Prevenzione study, however, which included lower doses of EPA but also DHA, the moderate dose of EPA alone in the JELIS trial was not associated with a reduction in SCD (possibly because of the virtual absence of SCD in this cohort).
In combination, the DART, GISSI-Prevenzione, and JELIS trials indicated that -3 PUFA lower CV risk in both primary and secondary prevention settings. However, it should be noted that other studies have not shown favorable results. For example, a trial by Burr et al. (19) suggested that patients with angina treated with fish oil capsules seem to have a higher risk of SCD than untreated control subjects. Von Schacky and Harris (20) criticized this trial as being suboptimally conducted or reported, thus the results are questionable. Also, a Norwegian study by Nilsen et al. (21) did not show a benefit of -3 PUFA supplementation in post-MI patients. Why treatment with -3 PUFA was successful in Japan but not in Norway, both populations with a high background intake of -3 PUFA, is not clear, but the much higher number in the former (n = 18,645) versus the latter (n = 300) was likely a factor. Finally, a recent trial (the OMEGA trial) was presented that assessed -3 PUFA (460 mg EPA + 380 mg DHA per day) for 1 year in 3,851 patients 3 to 14 days after acute MI from 104 centers in Germany (22). In these vigorously treated patients (85% to 95% usage of aspirin, clopidogrel, statins, beta-blockers, and angiotensin-converting enzyme inhibitors), the arrhythmic event rate and total mortality were only 0.7% and 3.7%, respectively, in the placebo group, and this trial showed no benefit of EPA/DHA on any of the primary or secondary end points. Although this study was probably underpowered to adequately determine the effects of this therapy in secondary CHD prevention, these preliminary results certainly raise the possibility that -3 PUFA may not provide additional short-term protection to low-risk patients receiving extensive modern post-MI therapies.
This review does not focus on ALA, which is found in abundance in flaxseed and to a lesser extent in canola and olive oil,walnuts, and other tree nuts, as well as trace amounts in green leafy vegetables. As the only dietary source of -3 PUFA, ALA is considered to be inadequate because humans convert typically <5% of ALA to EPA and even less to DHA (23). In some (but not all) epidemiologic studies, ALA has been inversely associated with CV events (3,24). For example, in a recent study from a Costa Rican population, ALA intake and blood levels predicted a better prognosis, independent of fish and EPA/DHA levels, in a post-MI population (25). Nevertheless, the overall evidence is much weaker for ALA than for EPA and DHA.
Evidence of Benefit in Atherosclerosis
Several epidemiologic and necropsy studies have indicated that Japanese men have significantly lower levels of atherosclerosis than Caucasian men residing in the U.S. (26,27). In studies that assessed intima-media thickness (IMT) and coronary artery calcification (CAC), both independent predictors of CV events, Japanese men have lower grades of atherosclerosis than do the Caucasians residing in the U.S. (26). Recently, Seikikawa et al. (27) have considered the "Japanese factor" in 281 Japanese men born and living in Japan, 281 Japanese men living in the U.S., and 360 Caucasian men born and living in the U.S. The overall results suggested that Japanese men had the lowest level of atherosclerosis, whereas Japanese in the U.S. and Caucasians had similar levels. The -3 PUFA serum levels correlated inversely with IMT in the Japanese men, but CAC burden was not related to -3 PUFA status in any cohort. Nevertheless, the differences between carotid IMT and CAC levels in the 3 groups, which persisted after adjustment for the traditional CHD risk factors, disappeared after adjustments for serum -3 PUFA content, suggesting that very high intake of marine-derived -3 PUFA has antiatherosclerotic effects (14,27). Despite these promising results, other evidence suggests that a very high intake of shortening and other saturated fats in the Westernized diet may overwhelm the beneficial effects of high -3 PUFA intake in Alaskan Natives and the Japanese (14).
Evidence in Arrhythmias
We (28) and others (29) have reviewed the antiarrhythmic effects of -3 PUFA. Chronic imbalance of the autonomic nervous system, with increases in symptomatic and/or decreases in parasympathetic tone, increases the risk of major CV events and dysrhythmias (3,28). Several randomized controlled trials show that -3 PUFA improve sympathovagal balance. Christenen et al. (30) found that patients post-MI and with impaired systolic function had improvements in heart rate variability after 4.3 g/day of EPA and DHA for 12 weeks. Using lower doses of -3 PUFA, O'Keefe et al. (31) showed significant reductions in resting heart rate, 1-min heart rate recovery after exercise, and improvement in heart rate variability after 4 months of modest-dose -3 PUFA(810 mg/day EPA and DHA). Geelen et al. (32) showed that 14 weeks of moderate-dose -3 PUFA (1,260 mg/day EPA and DHA) reduced the average heart rate in patients with complex ventricular arrhythmias. A large study of 5,096 men and women by Mozaffarian et al. (33) showed that high dietary fish intake was associated with lower heart rate, slower atrial ventricular conduction, and a substantially lower likelihood of having a prolonged QT interval. In aggregate, these studies suggest that -3 PUFA have benefits in improving autonomic function.
Current research suggests that -3 PUFA may prevent fatal arrhythmias via their ability to inhibit fast, voltage-dependent sodium channels and L-type calcium channels (28). Furthermore, DHA has been shown to directly inhibit the delayed-rectifier potassium channel, which is responsible for the depolarization phase of ventricular and atrial cardiac potentials. Although the relative effects of DHA and EPA remain uncertain, DHA's effect on atrial and ventricular repolarization raises the possibility that DHA could provide greater protection against dysrhythmias, a fact that is supported by the beneficial effects of combined EPA and DHA against SCD in the GISSI-Prevenzione trial (6) but not noted with higher doses of EPA alone in the JELIS trial (7).
Although -3 PUFA seem to be effective in reducing SCD in post-MI and in CHD patients with LV dysfunction (6,15-17), 3 trials using -3 PUFA in patients with implantable cardioverter-defibrillators (ICDs) have shown mixed results (34-36). The initial trial received substantial negative publicity when a subgroup of -3 PUFA-treated patients had more frequent ICD discharges compared with the placebo group, suggesting that these supplements may be proarrhythmic in certain patients (34). In another trial, Leaf et al. (35) found a trend for lower risk for the combined end point of ICD discharge + death from any cause (-28%; p = 0.057) in the group randomized to -3 PUFA, with risk reduction of close to 40% (p = 0.03) when adjusting for probable episodes of malignant arrhythmias and compliance. A third trial (and the largest) showed no significant differences between -3 PUFA and placebo in patients with ICD, but in a subgroup with prior MI, the -3 PUFA group had a trend toward benefit (p = 0.09) (36). These trials used 1.8, 2.6, and 0.8 g of -3 PUFA daily,respectively. The GISSI-HF study, discussed later, did not show any benefits against SCD with the same dose of -3 PUFA used in the GISSI-Prevenzione study, raising the possibility that -3 PUFA may not benefit SCD risk with large populations with significant LV dysfunction.
Perhaps the most significant antiarrhythmic effects, however, have been noted in studies of AF (28). Mozaffarian et al. (37) showed a 30% lower risk of AF over a 12-year follow-up in patients who consumed high quantities of nonfried fish. However, the Rotterdam study (38) found no such correlation. Two studies in patients undergoing coronary artery bypass grafting have suggested >50% reductions in the development of post-surgical AF in patients pre-treated with -3 PUFA with the number needed to treat being only 5.5 in one study (39); one of the studies also showed significant reduction in days hospitalized (39,40). Whether these benefits are caused by antiarrhythmic effects, benefits on autonomic tone, or even anti-inflammatory effects is impossible to determine from these trials. However, these trials point out the potential benefits of -3 PUFA in the current epidemic of AF.
Evidence of Benefit in HF
Recently, the potential benefits of -3 PUFA have been extended to the prevention and treatment of HF. The Cardiovascular Health Study, involving 4,738 men and women 65 years of age, found an inverse association of baked or broiled fish intake and incident congestive HF (Fig. 3) (41). This result was supported by recent data from the ARIC (Atherosclerosis Risk in Community) study, showing an inverse relationship between -3 PUFA intake and incident HF in women (42). A recent study by Yamagishi et al. (43) in a prospective study of nearly 60,000 Japanese followed up for nearly 13 years showed an inverse association between fish and -3 PUFA consumption and CV mortality, especially for HF. These results are particularly striking in a society with a comparatively high intake of fish and background -3 PUFA intake.
Confirmatory evidence was recently presented and published in the GISSI-HF trial (8), a large, factorial, placebo-controlled trial of nearly 7,000 patients with class II to IV HF who were randomized to 1 g of -3 PUFA (1 highly concentrated fish oil capsule, Lovaza, containing 850 to 882 mg of EPA + DHA), rosuvastatin (10 mg), both, or dual placebo. This large and well-done study showed a statistically significant benefit of the prescription -3 PUFA (Fig. 4), including reduction in total mortality (-9%; p < 0.05) and total mortality or hospitalizations for CV diseases (-8%; p < 0.01). Although these benefits seem to be only modest, they translate into 56 patients needing to be treated for 4 years to avoid 1 death or hospital CV admission. Importantly, this therapy was safe and well tolerated, and the improvements in clinical outcomes were additive to that of other well-established HF therapies, including beta-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blockers, and aldosterone receptor blockers. Unlike the GISSI-Prevenzione trial (6), which used the same dose of concentrated prescription -3 PUFA, prevention of SCD did not seem to explain the benefits of fish oil in HF, nor did HF hospitalizations account for these benefits.
Questions remain regarding the mechanisms responsible for the -3 PUFA effects in HF. In addition to the established effects of fish oil therapy, both EPA and DHA are potent activators of peroxisome proliferator-activator receptor (PPAR)-alpha (found in the heart) and PPAR-gamma (44). Although fatty acids are classically viewed as an energy substrate in the heart, they are also endogenous ligands for PPARs and regulate the expression of genes encoding key proteins controlling myocardial fatty acid uptake and metabolism (45). Stanley et al. (46) have shown that a high-fat diet increases plasma free fatty acid concentration, activating PPAR-alpha in the heart and stimulating expression of key mitochondrial proteins involving fatty acid oxidation. Duda et al. (47) observed that dietary -3 PUFA from fish oil (>1.6 g EPA + DHA) significantly increases serum levels of the cardioprotective adipokine adiponectin in rats subjected to either sham treatment or hypertension induced by abdominal aortic banding. Most importantly, the increase in adiponectin corresponded to significant attenuation of LV hypertrophy and correlated with decreased LV end-systolic volume. Recent evidence suggests that ligand activation of PPAR-gamma by EPA and/or DHA up-regulates adiponectin and suppression of inflammatory cytokines (48-50), which could improve cardiac structure and function in HF (51,52). Thus, important cardiac remodeling effects may underlie the observed clinical benefits of fish oils in HF.
In a small 18-week pilot study of 14 patients with class IIII to IV HF randomized to 5.1 g/day of EPA and DHA, we showed marked improvements in inflammatory cytokines (e.g., tumor necrosis factor alpha and interleukin-1), and percent body fat in advanced HF, suggesting that fish oil may be beneficial in decreasing inflammation and cachexia in advanced HF (53). This suggests a potentially novel therapeutic approach in the late stages of HF. In addition, our study raises the issue of the dose needed to obtain maximal clinical benefits in patients with HF. Both the GISSI-HF study (8) and the Japan epidemiological study (43) likewise raised the possibility that pharmacological doses may be needed in patients with HF. Animal studies in cardiac remodeling suggest the need for higher doses as well. Therefore, further studies are needed determining not only the optimal dose of -3 PUFA protection in different stages of HF but also the underlyingmechanisms accountable for their benefits. However, at present, we agree with Fonarow's (54) assertion that -3 PUFA supplementation "should join the short list of evidence-based life-prolonging therapy for HF."
Evidence for Benefit in Hyperlipidemia
The U.S. Food and Drug Administration (FDA) has approved an -3 PUFA ethyl ester formulation (Lovaza), at a dosage of 4 g/day for the treatment of very high triglyceride levels (500 mg/dl) (55-57). It is well established that -3 PUFA lower plasma triglyceride concentrations (20,58,59). The mechanism for theselipid-lowering effects seems to involve activation of PPARs. Although fatty acids are classically observed as an energy substrate, they are also endogenous ligands for PPARs and regulate the expression of genes encoding key proteins controlling fatty acid uptake and metabolism and the formation of very-low-density lipoproteins carrying triglycerides in the liver (60,61). Although the exact transcriptional mechanism by which fish oils improve lipid levels is not completely understood, -3 PUFA do reduce hepatic synthesis of triglycerides and increase hepatic fatty acid beta-oxidation. The triglyceride-lowering doses of DHA and EPA is 3 to 4 g/day. This dose typically reduces triglyceride levels by 30% to 40% (59) and has been shown to reduce severely elevated triglyceride levels (>500 mg/dl) by 45%, along with reductions in non-high-density lipoprotein cholesterol by 14% with a 9% increase in high-density lipoprotein cholesterol (62). When added to baseline statin therapy in patients with triglyceride levels between 200 and 499 mg/dl, this dosage of -3 PUFA lowers triglyceride levels by close to 30% (63). Generally, there are no significant improvements in levels of low-density lipoprotein (LDL) cholesterol with fish oil therapy, especially in patients with elevated triglyceride levels, who often notice increases between 5% and 50% (depending on the severity of the hypertriglyceridemia and baseline LDL levels) (62). Interestingly, in the JELIS study, moderate doses of EPA resulted in 10% reductions in LDL cholesterol beyond that produced by low-dose statins (7). Nevertheless, even when LDL cholesterol increases with -3 PUFA, as it can with fibrates and occasionally with niacin, -3 PUFA-enriched LDL has been reported to be larger and fluffier (pattern A), which is potentially less atherogenic than the smaller, denser (pattern B) LDL particles (64). Although typically more expensive than dietary supplements, the capsular form is a standardized prescription preparation (Lovaza, 4 g) with FDA-approved safety and efficacy data and is the most concentrated source of DHA and EPA available.
Additional Mechanisms and Optimal DHA/EPA Ratios
A detailed discussion of all of the potential mechanisms of -3 PUFA and CV diseases (summarized in Table 2) is beyond the scope of this review. It appears that -3 PUFA confer CV benefits largely through DHA and EPA enrichment of membrane phospholipids (65). In addition to mechanisms discussed above, -3 PUFA produces vasodilation, reduces blood pressure (31,66), improves arterial and endothelial function (67), and reduces platelet aggregation (68). The antiplatelet, anti-inflammatory, and triglyceride-lowering effects of -3 PUFA (Fig. 5) (69) require relatively higher doses of DHA and EPA (e.g., 3 to 4 g/day), whereas some of the antiarrhythmic effects, reduction of SCD, and improvement in HF can be achieved at lower doses (500 to 1,000 mg/day). Nevertheless, higher doses may be even more effective in HF, as discussed previously. Although the effects of -3 PUFA on C-reactive protein levels have been inconsistent (70), these agents have been shown to suppress production of pro-inflammatory cytokines such as interleukin-1B, interleukin-6, and tumor necrosis factor-alpha (71). When administered to obese patients, 1.8 g of EPA increased the levels of adiponectin, which can reduce inflammation and improve insulin sensitivity (72), in addition to the potential beneficial HF effects discussed earlier. Although benefits on the autonomic nervous system are well established and are reviewed earlier, studies in patients undergoing heart transplantation suggest that -3 PUFA can reduce heart rate independently of vagal activation (73), in addition to reducing mean arterial pressure and systemic vascular resistance by 25% and reducing LV hypertrophy and improving diastolic function in heart transplantation patients with cyclosporine-induced hypertension (66).
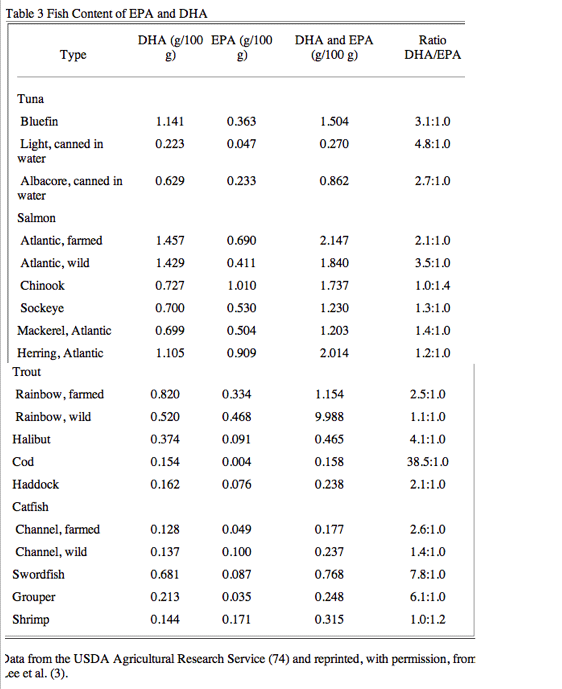
The optimal doses and ratios of DHA to EPA are difficult to decipher. Both DHA and EPA are present in most fish, particularly oily ones, generally in a 2:1 ratio (Table 3) (3,74), whereas fish oils typically have a ratio of 2:3 or lower (3). Although feeding pure DHA can raise EPA levels to a small extent (75), the reverse is not true (76). Additionally, DHA is far more abundant than EPA in the myocardium (68). As reviewed earlier, DHA alone or in combination with EPA may be more important for protection against dysrhythmias and SCD than EPA alone. Although the beneficial effects on dysrhythmias seem to occur at lower doses, the relative risk of SCD has been shown to be related with baseline blood levels of -3 PUFA (Fig. 6) (3,77) and, as reviewed earlier, protection against CHD was also inversely related with tissue levels of EPA and, more so, with DHA levels (15). In addition, other surrogate CV markers (arterial pressure, endothelial relaxation and attenuated vascular relaxation, and lipoproteins) may be more improved with high doses of DHA than with similar doses of EPA (78).
Safety and Adverse Effects
The most commonly observed adverse effects of -3 PUFA supplementation are nausea, gastrointestinal upset, and "fishy" burp. Prolonged bleeding times, as noted in Greenland Eskimos and when feeding "hyper-Eskimo" doses of -3 PUFA (e.g., over 20 g/day) to normal volunteers, usually remain in the high end of the normal range (3,10). These observations, however, raised concerns that higher intakes will increase hemorrhagic complications. However, Harris (79), in a comprehensive review, concluded that there was no increased risk of clinically significant bleeding noted with -3 PUFA doses of up to 7 g of combined DHA and EPA per day, even when combined with antiplatelet therapy or warfarin.
One of the major concerns, not about EPA and DHA per se, but about diets high in oily fish, is the consumption of contaminants, namely methyl mercury. For this reason, the FDA has advised children and pregnant or nursing women to specifically avoid those fish with a potentially high content of mercury, such as swordfish, tile fish, king mackerel, and shark (80). Nevertheless, a study of nearly 12,000 British women during their pregnancy and beyond found that women who exceeded the U.S. FDA recommendation for fish intake actually had offspring with better cognitive and behavioral development than offspring of women who consumed less fish during pregnancy (81). A large meta-analysis by Mozaffarian and Rimm (82) also showed the favorable risk-to-benefit ratio (1:400) associated with a high consumption of fish. Importantly, the most commonly consumed dietary sources of -3 PUFA, such as salmon, sardines, trout, oysters, and herring, are quite low in mercury (3). Because mercury is water soluble and protein bound, it is present in the muscle of the fish but not in the oil. Therefore, fish oil supplements should contain negligible amounts of mercury (83).
Recommendations
The current AHA dietary guidelines recommend combined EPA and DHA in a dose of approximately 1,000 mg/day in patients with CHD (9). This dose is largely determined from the GISSI-Prevenzione study, in which a dose of 850 mg was used. For those individuals without CHD, the AHA recommends 2 oily fish meals per week. This has been shown to be equivalent to about 500 mg/day of combined EPA and DHA, the intake associated with the lowest risk for CHD death in several U.S. prospective cohort studies (84). Several other major organizations, including the National Cholesterol Education Program (85), the World Health Organization (86), the European Society of Cardiology (87), the United Kingdom Scientific Advisory Committee in Nutrition (88), and the American Diabetes Association (89), have all provided guidelines that address increasing consumption of fish. Based on the GISSI-HF study results, we believe that these recommendations, similar to CHD, should also be extended to patients with HF (e.g., approximately800 to 1,000 mg of combined EPA/DHA daily) (8,54). In patients with hypertriglyceridemia, moderate to high doses of -3 PUFA (Lovaza, 4 g/day) is an FDA-approved therapy (55-57,64), and this therapy can be safely combined with any other lipid therapies (statins, fibrates, niacin, and so on). Further studies are needed that will determine the optimal dosing in various populations, especially those with HF, as well as the effects of various doses on the primary and secondary reduction of AF and more detailed mechanisms responsible for the benefits noted. Further studies will be needed to address the potential benefits of assessing blood levels of -3 PUFA to determine its role in dosing adjustments and its value in CV protection (90). Finally, studies are needed to determine the optimal mixture of DHA relative to EPA in various populations, as well as in light of the recent OMEGA trial (22), to determine the role of -3 PUFA in maximally treated contemporary post-MI patients or other patients with relatively low-risk CHD.
Conclusions
Convincing evidence from extensive research over the past 3 decades points out the potential beneficial effects of -3 PUFA in primary prevention, CHD and post-MI, SCD, HF, atherosclerosis, and AF. Based on the growing evidence for the benefits of fish oils, we agree that this story represents a "fish tale withgrowing credibility." We also agree with Rogans' comment from over 20 years ago that "fish oil is a whale of a story, that not surprisingly gets bigger with every telling" (1).
references
1. Rogans JA. More on fish oil N Engl J Med 1987;316:624-628
2. Lavie CJ, Milani RV. Fish oilsIn: Messerli FH, editor. Cardiovascular Drug Therapy. Philadelphia: Saunders; 1996. pp. 1608-1613.
3. Lee JH, O'Keefe JH, Lavie CJ, Marchioli R, Harris WS. Omega-3 fatty acids for cardioprotection Mayo Clin Proc 2008;83:324-332.
4. Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease N Engl J Med 1985;312:1205-1209.
5. Albert CM, Hennekens CH, O'Donnell CJ, et al. Fish consumption and risk of sudden cardiac death JAMA 1998;279:23-28.
6. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico -errata in Lancet 2001;357:642 and Lancet 2007;369:106] Lancet 1999;354:447-455
7. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis -published correction appears in Lancet 2007;370:220] Lancet 2007;369:1090-1098
8. GISSI-HF Investigators Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial Lancet 2008;372:1223-1230.
9. Kris-Etherton PM, Harris WS, Appel LJ, American Heart Association Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease -published correction appears in Circulation 2003;107:512] Circulation 2002;106:2747-2757.
10. Sinclair HM. The diet of Canadian Indians and Eskimos Proc Nutr Soc 1953;12:69-82.
11. Bang HO, Dyerberg J. Plasma lipids and lipoproteins in Greenlandic west coast Eskimos Acta Med Scand 1972;192:85-94.
12. Bang HO, Dyerberg J. Lipid metabolism and ischemic heart disease in Greenland Eskimos Adv Nutr Res 1980;3:1-22.
13. Dyerberg J, Bang HO, Hjorne N. Fatty acid composition of the plasma lipids in Greenland Eskimos Am J Clin Nutr 1975;28:958-966.-
14. William H. Omega-3 fatty acids: the "Japanese" factor? J Am Coll Cardiol 2008;52:425-427.
15. Harris WS, Poston WC, Haddock CK. Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events Atherosclerosis 2007;193:1-10.
16. Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: Diet And Reinfarction Trial (DART) Lancet 1989;2:757-761.
17. Macchia A, Levantesi G, Franzosi MG, et al. Left ventricular systolic dysfunction, total mortality, and sudden death in patients with myocardial infarction treated with n-3 polyunsaturated fatty acids Eur J Heart Fail 2005;7:904-909.-
18. Marchioli R, Barzi F, Bomba E, et al. , for the GISSI-Prevenzione Investigators. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002;105:1897-1903.-
19. Burr ML, Ashfield-Watt PA, Dunstan FD, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial Eur J Clin Nutr 2003;57:193-200.
20. von Schacky C, Harris WS. Cardiovascular benefits of omega-3 fatty acids Cardiovasc Res 2007;73:310-315.-
21. Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol Am J Clin Nutr 2001;74:50-56.-
22. Senges S, OMEGA Study Group Randomized trial of omega-3 fatty acids on top of modern therapy after acute myocardial infarction: the OMEGA trial. Oral presentation at: Annual Scientific Sessions of the American College of Cardiology. March 2009Orlando, FL.
23. Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements Appl Physiol Nutr Metab 2007;32:619-634.
24. Wang C, Harris WS, Chung M, et al. n-3 fatty acid from fish or fish-oil supplements, but not -alpha]-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review Am J Clin Nutr 2006;84:5-17.-
25. Campos H, Baylin A, Wilett WC. -linolenic acid and risk of nonfatal acute myocardial infarction Circulation 2008;118:339-345.-
26. Seikikawa A, Ueshima H, Kadowaki T, et al. Less subclinical atherosclerosis in Japanese men in Japan than in white men in the United States in the post-World War II birth cohort Am J Epidemiol 2007;165:617-624.-
27. Seikikawa A, Curb JD, Ueshima H. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study J Am Coll Cardiol 2008;52:417-424.-
28. Anand RG, Alkadri M, Lavie CJ, Milani RV. The role of fish oil in arrhythmia prevention J Cardiopulm Rehabil 2008;28:92-98.
29. Reiffel JA, McDonald A. Antiarrhythmic effects of omega-3 fatty acids Am J Cardiol 2006;98:501-601.
30. Christensen JH, Gustenhoff P, Korup E, et al. Effect of fish oil on heart rate viability in survivors of myocardial infarction: a double blind randomized controlled trial Br Med J 1996;312:677-678.
31. O'Keefe Jr. JH, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate viability in men with healed myocardial infarctions and depressed ejection fractions Am J Cardiol 2006;97:1127-1130.
32. Geelen A, Brouwer IA, Schouten EG, Maan AC, Katan MB, Zock PI. Effects of n-3 fatty acids from fish on premature ventricular complexes and heart rate in humans Am J Clin Nutr 2005;81:416-420.-
33. Mozaffarian D, Prineas RJ, Stein PK, Siscovick DS. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans J Am Coll Cardiol 2006;48:478-484.-
34. Raitt MH, Connor WE, Morris C, et al. Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial JAMA 2005;293:2884-2891.-
35. Leaf A, Albert CM, Josephson M, et al. Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake Circulation 2005;112:2762-2768.-
36. Brouwer IA, Zock PL, Camm AJ, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial JAMA 2006;295:2613-2619.-
37. Mozaffarian D, Psaty BM, Rimm EB, et al. Fish intake and risk of incident atrial fibrillation Circulation 2004;110:368-373.-
38. Brouwer IA, Heeringa J, Geleijnse JM, Zock PL, Witteman JC. Intake of very long-chain n-3 fatty acids from fish and incidence of atrial fibrillation. The Rotterdam Study. Am Heart J 2006;151:857-862.
39. Calo L, Bianconi L, Colicicchi F, et al. N-3 Fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: a randomized, controlled trial J Am Coll Cardiol 2005;45:1723-1728.-
40. Crystal E, Garfinkle MS, Conolly SS, Ginger TT, Sleik K, Yusuf SS. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery Cochrane Database Syst Rev 2004;4CD003611.
41. Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure J Am Coll Cardiol 2005;45:2015-2021.-
42. Yamagishi K, Nettleton JA, Folsom AR. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk In Communities (ARIC) study Am Heart J 2008;156:965-974.
43. Yamagishi K, Iso H, Date C, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women J Am Coll Cardiol 2008;52:988-996.-
44. Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferators-activated receptors alpha and gamma Proc Natl Acad Sci U S A 1997;94:4318-4323.-
45. Xu HE, Lambert MH, Montana VG, et al. Molecular recognition of fatty acids by peroxisome proliferators-activated receptors Mol Cell 1999;3:397-403.
46. Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart Physiol Rev 2005;85:1093-1129.-
47. Duda MK, O'Shea KM, Lei B, et al. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload Cardiovasc Res 2007;76:303-310.-
48. Neschen S, Morino K, Rossbacker JC, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice Diabetes 2006;55:924-928.
49. Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease Circ Res 2005;96:939-949.-
50. Itoh M, Suganami T, Satoh N, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects Arterioscler Thromb Vasc Biol 2007;27:1918-1925.-
51. Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through Nat Med 2005;11:1096-1103.
52. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor Clin Chim Acta 2007;380:24-30.]
53. Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improved body weight in severe heart failure J Heart Lung Transplant 2006;25:834-838.
54. Fonarow GC. Statins and n-3 fatty acid supplementation in heart failure Lancet 2008;372:1195-1196.
55. Bays H. Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids Am J Cardiol 2006;98:71i-76i.
56. Bays H. Rationale for prescription omega-3-acid ethylester therapy for hypertriglyceridemia: a primer for clinicians Drugs Today (Barc) 2008;44:205-246.
57. Bays H, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications Expert Rev Cardiovasc Ther 2008;6:391-409.]
58. Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease Circulation 2002;106:2747-2757.
59. Harris WS. N-3 fatty acids and serum lipoproteins: human studies Am J Clin Nutr 1997;65(Suppl):1645S-1654S.
60. Berger J, Moller DE. The mechanisms of action of PPARs Annu Rev Med 2002;53:409-435.
61. Huang B, Wu P, Bowker-Kinley MM, Harris RA. Regulation of pyruvate dehydrogenase kinase expression by peroxisome proliferator-activated receptor-alpha ligands, glucocorticoids, and insulin Diabetes 2002;51:276-283.
62. Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia J Cardiovasc Risk 1997;4:385-391.]
63. Davidson MH, Stein EA, Bayes HE, et al. , for the COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/day to simvastatin 40 mg/day in hypertriglyceridemic patients: an 8-week randomized, double-blind, placebo-controlled study. Clin Ther 2007;29:1354-1367.
64. Harris WS, Jacobson TA. Omega-3 fatty acidsIn: Ballantyne CM, editor. Clinical Lipidology: A Companion to Braunwald's Heart Disease. Philadelphia, PA: Saunders, an imprint of Elsevier Inc.; 2009. pp. 326-338.
65. Harris WS. Omega-3 fatty acids and cardiovascular disease a case for omega-3 index as a new risk factor Pharmacol Res 2007;55:217-223.
66. Ventura HO, Milani RV, Lavie CJ, et al. Cycosporine-induced hypertension: efficacy of omega-3 fatty acids in patients after cardiac transplantation Circulation 1993;88:11281-11285.
67. Theis F, Garry JM, Yaquoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomized controlled trial Lancet 2003;3161:477-485.
68. Din JN, Harding SA, Valerio CJ, et al. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man Atherosclerosis 2008;197:290-296.
69. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits -errata in JAMA 2007;297:5091] JAMA 2006;296:1885-1899.-
70. Madsen T, Schmidt EB, Christensen JH. The effect of n-3 fatty acids on C-reactive protein levels in patients with chronic renal failure J Ren Nutr 2007;17:258-263.
71. Zhao G, Etherton TD, Martin KR, Gilles PJ, West SG, Kris-Etherton PM. Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects Am J Clin Nutr 2007;85:385-391.-
72. Itoh M, Suganami T, Satoh N, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects Arterioscler Thromb Vasc Biol 2007;27:1918-1925.-
73. Harris WS, Gonzales M, Laney N, Sastre A, Borkon AM. Effects of omega-3 fatty acids on heart rate in cardiac transplant recipients Am J Cardiol 2006;98:1393-1395.
74. USDA Agricultural Research Service Nutrient Data Laboratory http://www.ars.usda.gov/nutrientdata 2006Accessed January 23, 2008.
75. Conquer JA, Holub BJ. Dietary docosahexaenoic acid as a source of eicosapentaenoic acid in vegetarians and omnivores Lipids 1997;32:341-345.
76. Park Y, Harris WS. Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance J Lipid Res 2003;44:455-463.-
77. Albert CM, Campos H, Stampfer MJ, et al. Blood levels of long-chain n-3 fatty acids and the risk of sudden death N Engl J Med 2002;346:1113-1118.-
78. Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans Curr Opin Clin Nutr Metab Care 2006;9:95-104.
79. Harris WS. Expert opinion: omega-3 fatty acids and bleeding-cause for concern? Am J Cardiol 2007;99:44C-46C.
80. U.S. Food and Drug Administration, Department of Health and Human Services Backgrounder for the 2004 FDA/EPA Consumer Advisory: What you Need to Know About Mercury in Fish and Shellfishhttp://www.fda.gov/oc/opacom/hottopics/mercury/backgrounder.html 2007Accessed January 24, 2008.
81. Hibbeln JR, Davis JM, Steer C, et al. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood. ALSPCA study: an observational cohort study. Lancet 2007;369:578-585.
82. Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits -errata in JAMA 2007;297:5901] JAMA 2006;296:1885-1899.-
83. Foran SE, Flood JG, Lewandrowski KB. Measurement of mercury levels in concentrated over-the-counter fish oil preparations: is fish oil healthier than fish? Arch Pathol Lab Med 2003;127:1603-1605.
84. Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults Curr Atheroscler Rep 2008;10:503-509.]
85. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) JAMA 2001;285:2486-2497.
86. World Health Organization Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Technical report No. 916. Geneva: World Health Organization; 2003.
87. DeBacker G, Ambrosioni E, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J 2003;24:1601-1610.
88. UK Scientific Advisory Committee on Nutrition Advice on Fish Consumption Benefits and RisksLondon: The Stationery Office; 2004.
89. Franz MJ, Bantle JP, Beebe CA, et al. Nutrition principles and recommendations in diabetes Diabetes Care 2004;27(Suppl 1):S36-S46.]
90. Harris WS. The omega-3 index as a risk factor for coronary heart disease Am J Clin Nutr 2008;87:1997S-2002S.-
|
|
| |
| |
|
|
|