 |
 |
 |
| |
Differences in Virologic Response Among African Americans and Females Regardless of Therapy in the HEAT Study: African-Americans Have Lower Viral Response Rates
|
| |
| |
Reported by Jules Levin
IAS 2009- 5th Conference on HIV Pathogenesis, Treatment and Prevention 19-22
July 2009, Cape Town South Africa
Smith KY1, Kumar PN2, Patel P3, Bellos NC4, Sloan L5, Lackey P6, Sutherland-Phillips DH3, Vavro C3, Yau L3, Shaefer MS3
1Rush University Medical Center, Chicago, IL USA; 2Georgetown University, Washington, DC, USA; 3GlaxoSmithKline, Research Triangle Park, NC, USA;
4Southwest Infectious Disease Associates, Dallas, TX, USA; 5North Texas Infectious Disease Consultants, Dallas, TX, USA; 6ID Consultants, Charlotte, NC, USA
AUTHOR DISCUSSION
Historically, under-representation of women and minorities in clinical trials has hampered our ability to critically examine differences in virologic and immunologic response in these vulnerable subgroups.3 In the HEAT study enrollment of women was nearly 20% and minorities made up more than 55% of the study population. Thus, this study provides an opportunity to assess treatment responses in these populations.
In this analysis, women appeared to have a reduced virologic response rate compared to the overall population, and this difference was seen in both treatment groups. However, CD4+ cell responses were robust especially in the ABC/3TC group. Despite lower virologic response rates, few women experienced protocol-defined virologic failure.
Fewer African-American subjects achieved virologic suppression compared to the overall population and African-American race was a significant predictor of virologic failure in a multivariate logistic regression model. CD4+ cell responses among African-Americans were not different compared to the overall population.
Reduced treatment responses among women and African-American subjects was also observed in the KLEAN study (FPV/r vs. LPV/r each with ABC/3TC) of 878 treatment-naive subjects through 48 weeks.4
AUTHOR CONCLUSIONS
Females and African-Americans had reduced treatment response rates compared to the overall and subgroup populations through 96 weeks.
African-American race, but not female sex, was a significant predictor of virologic failure in a multivariate logistic regression model.
Greater CD4+ responses were noted among females in the ABC/3TC group; no treatment differences in CD4+ response were seen among African-Americans in this study.
Large comparative trials of traditionally under-represented populations are needed to further investigate these differences in virologic and immunologic outcomes.
ABSTRACT
Objectives: Differences in virologic and immunologic response have been observed with different antiretroviral combinations. We evaluated week 96 responses by sex and by race in the HEAT study which previously demonstrated the non-inferiority of abacavir sulfate/lamivudine (ABC/3TC) versus tenofovir/emtricitabine (TDF/FTC), when each was combined with lopinavir/ritonavir (LPV/r), at 48 weeks and subsequently confirmed at 96 weeks in ART-na´ve subjects.
Methods: Virologic response at week 96 was determined by the proportion with HIV-1 RNA (VL) <50 c/mL by missing=failure (M=F) and observed analyses. Race and ethnicity were self-identified.
Results: Immunologic and Virologic Response at Week 96
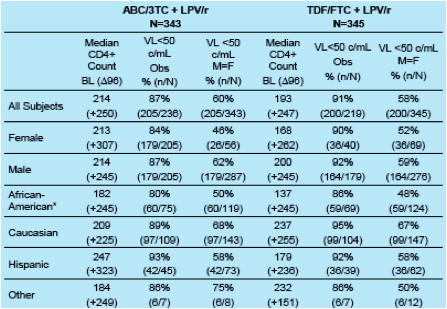
*African-American race, but not female sex, was a significant predictor of virologic failure in a multivariate logistic regression model studied.
Conclusions: Differences in CD4+ and virologic responses by sex and race at week 96 were observed. Notably, fewer African-Americans and females achieved VL <50 c/mL in both arms and in both analyses suggesting these differences may be multifactorial. Slightly greater CD4+ increases were observed in females and although African-Americans had lower initial CD4 counts, immunologic recovery was similar across other racial groups and between groups over 96 weeks. Further investigation into the underlying differences in response is
required.
INTRODUCTION
Differences in response to antiretroviral therapy appear to be associated with numerous factors including sex and subgroups of race/ethnicity. 1
HEAT, a randomized, double-blind, placebo-matched, multicenter trial demonstrated the non-inferiority of abacavir sulfate/lamivudine (ABC/3TC) to tenofovir/emtricitabine (TDF/FTC), each in combination with once-daily lopinavir/ritonavir (LPV/r), in ART-na´ve subjects over 96
weeks2
We investigated virologic and immunologic response by sex and racial sub-groups in the HEAT study.
METHODS
688 subjects were included in the intent-to-treat-exposed (ITT-E) population from sites across the United States and Puerto Rico.
The primary endpoint was the proportion of subjects with HIV-1 RNA <50 copies/mL (c/mL) at Week 48 by Missing=Failure (M=F) analysis. Subjects that switched off randomized treatment were included (Switch included).
Subjects self-identified race and ethnicity separately. Efficacy outcomes were summarized for all subjects in the ITT(E) population, as well as, by sex (female or male) and by race (african-american, caucasian, hispanic ethnicity or other race).
No formal assessment of medication compliance was performed in this
study.
No formal statistical comparisons were performed in this sub-group
analysis.
RESULTS
Table 1. Baseline Demographics of ITT-E Population
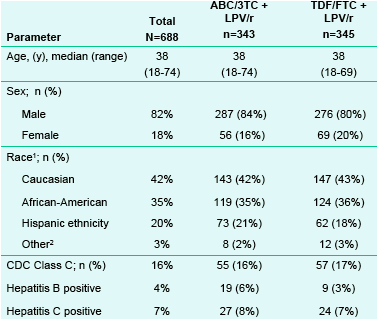
1. For this race summary, subjects were grouped based on self-reported ethnicity, and for subjects with non-Hispanic ethnicity, their self-identified race were shown.
2. Other represents Asian, American Indian/Alaskan Native, or another race
The ITT-E population was balanced between treatment groups. Fewer than 20% of the population was represented by female subjects. However, 49% of the population was represented by non-Caucasian subjects of which 36% were African-American.
Table 2. Median Baseline Viral Load and CD4+ Count
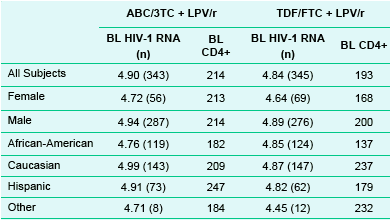
Females had generally lower baseline viral loads in both groups and only African-Americans in the ABC/3TC + LPV/r group compared to the overall population and other sub-groups. CD4+ counts were generally lower at baseline in African-American subjects as well.
Figure 1. Proportion of Subjects with HIV-1 RNA <50
c/mL at Week 96; ITT(E) Population, M=F Analysis
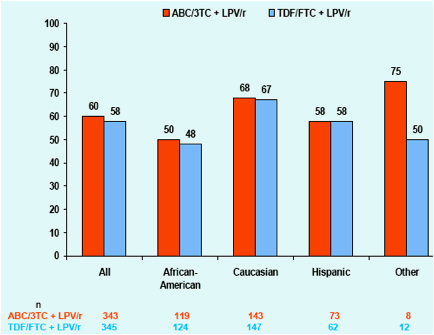
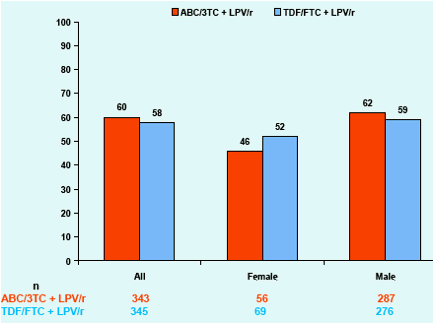
The percentage of subjects with an HIV-1 RNA <50 copies/mL at Week 96 was somewhat lower among female and African-American subjects compared to the overall population and other sub-groups.
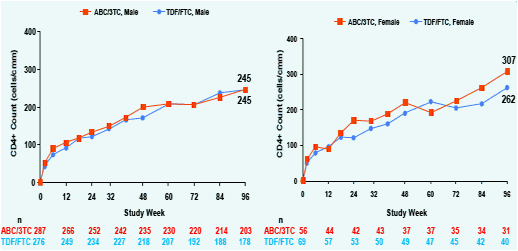
Figure 2. Change from baseline in CD4+ Count at
Week 96; ITT(E) Population, Observed Analysis
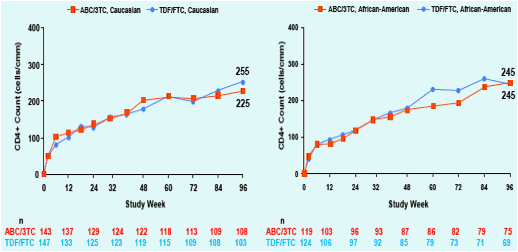
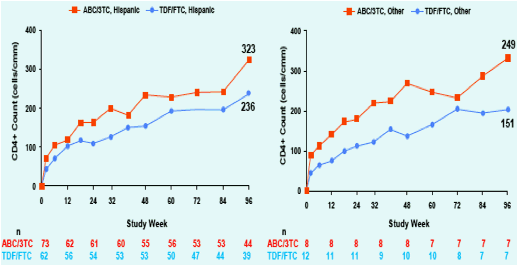
Overall, female subjects tended to have greater CD4+ recovery compared to males. CD4+ recovery appeared similar between treatment arms within the race and ethnicity sub-groups. Small sample sizes prevented any statistical comparison.
REFERENCES
1. Tashima K, Kumar P, Rodriguez-French A, et al. Gender and race subgroup analyses in 4 large, randomized clinical trials comparing abacavir (ABC) to protease inhibitors (PI) or zidovudine (ZDV) in ART-na´ve subjects. 8th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; 2006 September 24-26; San Francisco, CA, USA.
2. Smith KY, Patel P, Fine D, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS 2009;published ahead of print, June 23 2009.
3. UNAIDS/WHO. AIDS Epidemic Update, December 2007.
4. DeJesus E, Gathe J, Katlama C, et al. Virologic response and tolerability by sex and race in subjects receiving fosamprenavir/ritonavir (FPV/r) BID and lopinavir/ritonavir (LPV/r) BID, each in combination with abacavir/lamivudine QD (the KLEAN study). 4th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 2007 July 22-25; Sydney, Australia.
|
| |
|
 |
 |
|
|