 |
 |
 |
| |
Effects of CYP2B6 Single Nucleotide Polymorphisms (SNPs) and Substance Abuse on Efavirenz (EFV) Pharmacokinetics
|
| |
| |
Reported by Jules Levin
ICCAC Sept 11 2009 San Francisco
From Jules: As I said yesterday in the similar report on Reyataz I'm not convinced of the plausibility of the findings. I asked if viral failure was associated and I think I recall but I'm not sure the authors said no, this is an initial study to explore the concept and a further followup is I think planned. In table 1 below the differences of the affect on viral load between substance users and non-users was not statistically significant, although both tobacco & alcohol use are statistically significant in reducing EFV trough. A question is this affect related to how much tobacco and alcohol is consumed, is their an effect for patients with high levels of consumption and not for patients with low levels, so does the quantity of consumption drive the results. Does smoking 3 packs daily of cigarettes drive the results, and you could ask the same about alcohol use, Another question is the effect of alcohol due to affecting liver metabolism, do these patients have cirrhotic livers and does that cause the effect. The question of adherence in these patients raised a concern to me but I asked the authors and they felt the patients were adherent. Patients using substances like these could have other challenges that reduce adherence and the results could be a surrogate for that but the authors feel relatively confident of their findings. A good question is what is the impact of this information if patients and clinicians hear about it, will this affect their commitment to HAART or affect their use of these substances, perhaps convincing patients not to use them since they could affect the success of HAART and health and longevity. Frankly, I don't have an answer for that but since this data has been reported I think further discussion and analysis is important to see how reliable this data is and further analysis of other PIs, NNRTIs and NRTIs ought to be conducted to see if the same affect occurs. I asked the authors why they selected EFV & ATV for their first study and they said it was because these are the most widely used ARTs. The poster does not say if patients were taking EFV or Atripla but the author said they think all were on EFV but they are verifying this. Another dataset on lopinavir is being analyzed.
C Venuto1, Q Ma1, D Brazeau1, B Zingman2, R Reichman3, M Fischl4, B Gripshover5, R DiFrancesco1, A Forrest1, G D Morse1
1Univ at Buffalo, Buffalo, NY; 2Montefiore Med Ctr, Einstein-Montefiore Ctr for AIDS Res, Bronx, NY; 3Univ of Rochester, Rochester, NY; 4Univ of Miami, Miami, FL; and 5Case Western Reserve Univ, Cleveland
AUTHOR CONCLUSIONS
These data confirm the previously reported association between CYP2B6 516G>T and efavirenz pharmacokinetics.
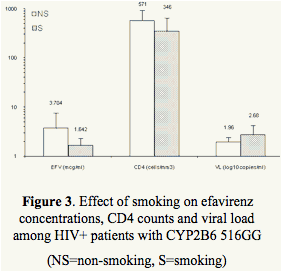
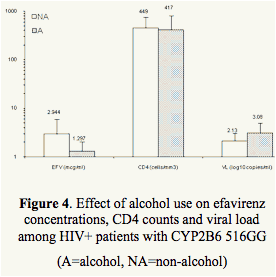
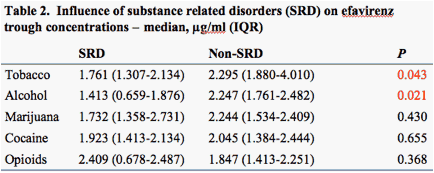
Tobacco and alcohol use is inversely related to efavirenz trough concentrations among patients with functional allele (GG) and was associated with lower CD4 counts and higher viral loads.
The mechanisms that underlies these observations may include both pharmacogenomic and substance related disorder components.
Clinicians should consider these pharmacologic findings when developing ART regimens for HIV+ patients with substance related disorders.
ABSTRACT
Background: Single nucleotide polymorphisms (SNPs) in CYP2B6 influence efavirenz pharmacokinetics. Our objective was to further evaluate the relationship of SNPs to EFV concentrations in HIV+ patients with substance-related disorders (SRDs).
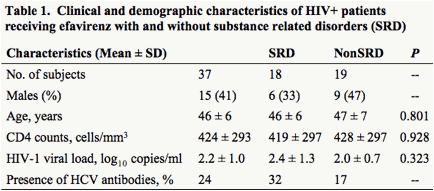
Methods: 37 HIV+ individuals with (n=18) or without (n=19) SRDs on EFV-containing regimens were enrolled. The primary analysis involved CYP2B6 516G>T which is associated with plasma EFV exposure. Secondary analyses included SNPs in ABCB1, CYP3A4, and 3A5. The association between genotypes and substances of abuse, viral load, and CD4 cell counts was evaluated using Kruskal-Wallis and linear regression tests.
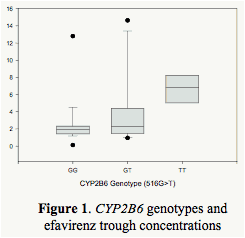
Results: Based on CYP2B6 516G>T genotypes, the patients were categorized as extensive (GG, n=19), intermediate (GT, n=13), and slow (TT, n=5) metabolizers. These genotypes with 2B6 were significantly associated with trough EFV concentrations (p=0.036), but not with ABCB1, 3A4 or 3A5. Significantly lower EFV concentrations were noted in tobacco and alcohol users in the extensive metabolizer group with lower CD4 counts and higher viral loads. SRD had no significant relationship to antiviral responses.
Conclusions: In addition to an association between CYP2B6 516G>T and EFV pharmacokinetics, tobacco and alcohol use was associated with significantly lower EFV trough concentrations among patients with functional alleles. The mechanisms that underlie these observations may include combined pharmacogenomic and behavioral components.
BACKGROUND
Efavirenz (EFV) is considered a first-line drug for a non-nucleoside reverse transcriptase inhibitor-based regimen in the initial treatment of HIV infection. Despite the fixed daily dose of 600 mg EFV in adults, it is characterized by significant interindividual pharmacokinetic variability resulting in variable response across patients. Central nervous system toxicities reflect elevated EFV plasma concentrations >4 µg/mL. Conversely, patients with an insufficient exposure to EFV (<1 µg/mL) are at increased risk of virologic resistance and treatment failure.
EFV is primarily metabolized by the polymorphic cytochrome P450 2B6 (CYP2B6) enzyme. The CYP2B6*6 (516G>T and 785A>G) allele results in reduced levels of protein expression and activity, and has been associated with increased plasma EFV levels.
Individuals with substance-related disorders (SRDs) have impaired adherence and imposed barriers to antiviral access. As a substrate and inducer of CYP2B6 and CYP3A4, EFV may interact with substances of abuse such as tobacco, alcohol, and marijuana through various mechanisms.
STUDY OBJECTIVE
To further evaluate the relationship of SNPs to EFV concentrations in HIV-infected patients with substance-related disorders.
To identify substances of abuse that have significant impact on EFV concentrations.
METHODS
Study subjects were participants in a multicenter therapeutic drug monitoring and drug interactions protocol for HIV-infected individuals with or without SRDs. Enrollment was from May 15, 2003 to May 15, 2007 at 4 clinical sites including Bronx, NY; Rochester, NY; Miami, FL and Cleveland, OH. The substances of abuse included cigarettes, alcohol, marijuana, cocaine, and methadone. Each subject was instructed to complete three clinic visits, entry, trough, and directly observed therapy (DOT), separated by 1-2 week intervals. The substance abuse status was determined by clinicians at entry visit. Subjects were counseled to take scheduled doses of efavirenz (600 mg once daily) at the same time for 4 days prior to each visit. Plasma samples were collected for drug assay and pharmacokinetic evaluation following adherence assessment and counseling. Laboratory data e.g. CD4+ cell counts and HIV RNA were recorded during the clinic visits.
Genotyping: DNA extraction was performed using the QIAGEN DNA Mini Blood Kit (QIAGEN, Valencia, CA). CYP2B6 polymorphism (516G>T in exon 4, CYP2B6*6) was identified by real-time PCR assay using Stratagene Mx4000TM (Stratagene, La Jolla, CA) with specific TaqMan® probes (Applied Biosystem, Foster City, CA) to recognize the wild and mutant alleles.
Drug assay: Plasma EFV concentrations were measured using a HPLC method previously developed, validated, and certified by the New York State Department of Health at Core Analytical Laboratory, University at Buffalo.
Statistical analysis: Continuous variables were compared by the Kruskal-Wallis test, and categorical variables were compared by the chi-square and Fisher's exact tests. Multiple linear regression models were used to determine factors associated with EFV concentrations, immunological and virologic responses while adjusting for covariates.
RESULTS
Baseline characteristics are summarized in Table 1. No significant difference was noted between SRD and non-SRD groups in age, CD4 counts or HIV-1 viral load.
For 516G>T genotype, the patients were categorized as extensive (GG, n=19), intermediate (GT, n=13), and slow (TT, n=5) metabolizers. The genotype was significantly associated with trough EFV concentrations adjusted with age, race, sex and BMI (p=0.036, Figure 1).
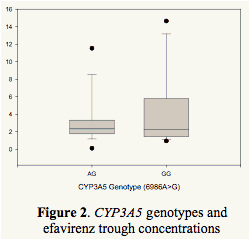
ABCB1 or CYP3A5 genotypes had no significant impact on EFV trough concentrations (Figure 2).
Significantly lower EFV concentrations were noted in tobacco and alcohol users in the extensive metabolizer group (GG) with lower CD4 counts and higher viral loads (Table 2, Figures 3 and 4).
SRD had no direct relationship to antiviral responses.
|
| |
|
 |
 |
|
|