 |
 |
 |
| |
Metabolic Profiles and Body Composition Changes in Treatment-Naïve HIV-Infected Patients Treated with Raltegravir 400 mg bid-based vs. Efavirenz 600 mg qhs-based Combination Therapy: 48-Week Data
|
| |
| |
Reported by Jules Levin
ICAAC Sept 14 2009
E. DeJesus1, A. Lazzarin2, J. Lennox3, D. Berger4, R. Pollard5, J. Madruga6, J. Zhao7, A. Rodgers7, B-Y. Nguyen7, R. Leavitt7, P. Sklar 7 for the STARTMRK (P021) Investigators
1Orlando Immunology Center, Orlando, FL, USA; 2University Vita-Salute San Raffaele, Milan, Italy; 3Emory University, Atlanta, GA, USA; 4Northstar Medical Center, University of Illinois at Chicago, Chicago, IL, USA;
5University of California @ Davis, Sacramento, CA, USA; 6Centro de Referencia e Treinamento DST/AIDS, Sao Paulo, Brazil; 7Merck Research Labs, North Wales, PA, USA
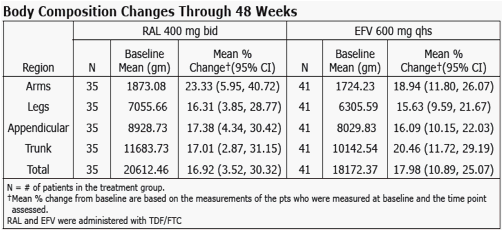
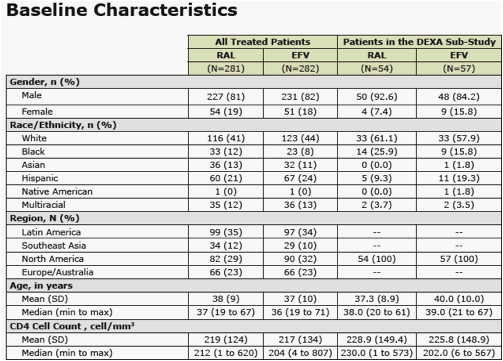
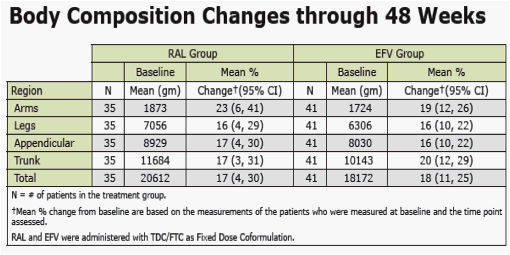
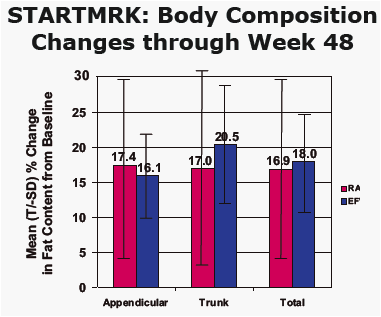
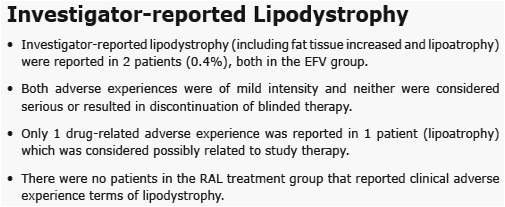
from Jules: Merck presented here body changes and metabolic changes (lipids, glucose) after 48 weeks for patients taking EFV or raltegravir. the dexa substudy was 35 patients. Mean body changes through 48 weeks showed similar increased fat in all body parts (see table in report) for both EFV & RAL. Baseline CD4 for the entire study was just over 200 in all groups. In the dexa substudy a significant percent of patients had high viral load and 15% had <50 CD4. Previous research showed patients with advanced HIV could be more likely to develop body changes but that was when we were using the older nukes. In this study investigator-reported lipodystrophy were reported in 2 patients. So we will have to wait until we see the 48-96 weeks data to see if there are changes. In previous studies ART-naive patients put weight on during the first year of therapy. We also know HIV can contribute to fat loss. The lipids and glucose profile looks good, see table in report.
ABSTRACT
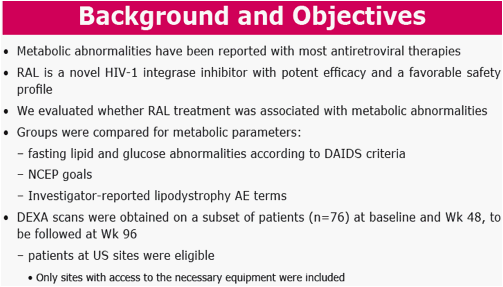
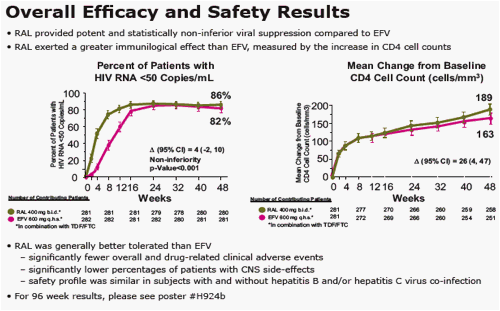
Background: RAL is a 1st in class integrase strand-transfer inhibitor. Metabolic parameters were compared between RAL-based and EFV-based regimens after 48 wks of treatment.
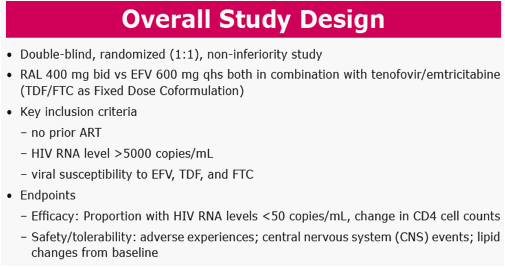
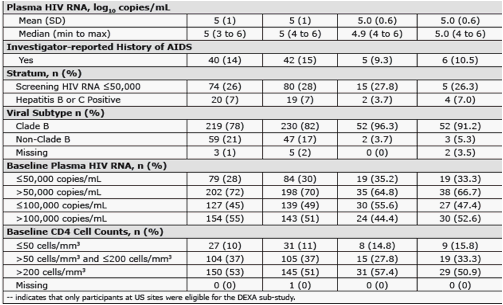
Methods: Pts were randomized in a double-blind study of RAL vs EFV, each with TDF/FTC (n=563). Groups were compared for metabolic parameters, including fasting lipid and glucose (glc) abnormalities according to DAIDS criteria, NCEP goals, and for reported lipodystrophy AE terms. DEXA scans were obtained on a subset of pts (n=76) at baseline and Wk 48, to be followed at Wk 96.
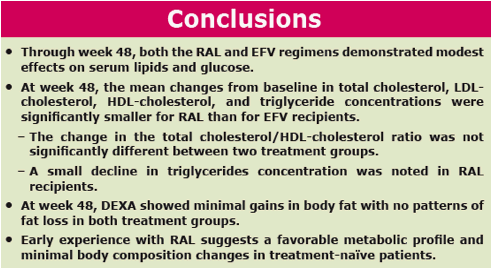
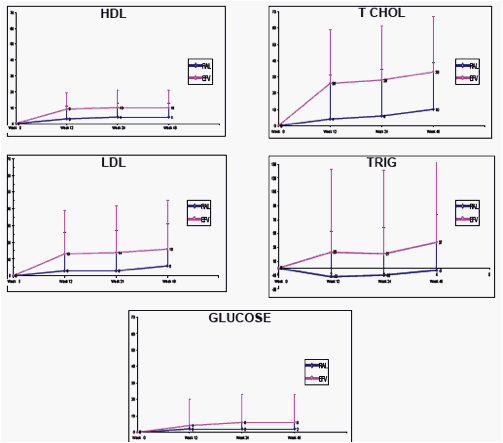
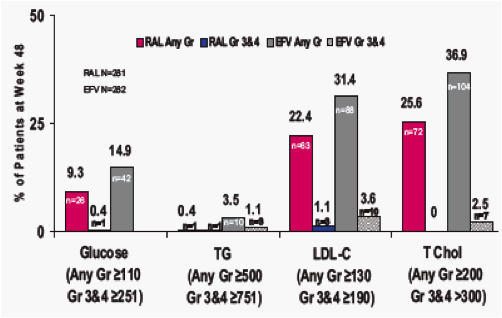
Results: At Wk 48, changes from baseline cholesterol (C), LDL-C, & triglycerides were lower in RAL vs EFV recipients (each p<0.001); HDL-C was higher in the EFV group (p<0.001). 26/281 on RAL and 42/282 on EFV had fasting serum glc of any grade (1-4); 1/26 on RAL was grade 3. AE of mild lipodystrophy were reported in 2 pts, both on EFV.
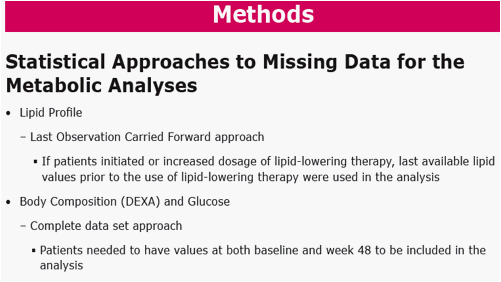
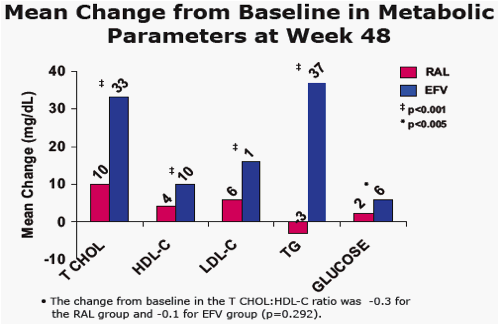
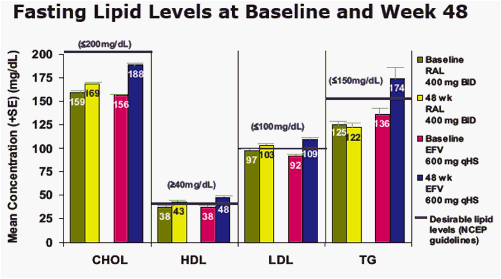
Mean Change from Baseline in Metabolic Parameters
DAIDS-Graded Metabolic Abnormalities at Week 48
|
| |
|
 |
 |
|
|