 |
 |
 |
| |
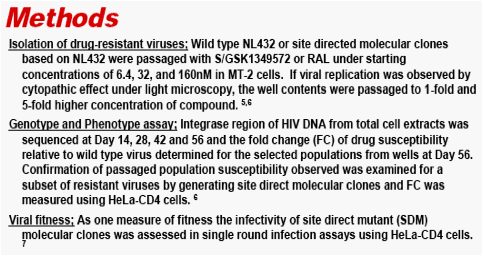
In Vitro Passage of Drug Resistant HIV-1 against a Next Generation Integrase Inhibitor (INI), S/GSK1349572
|
| |
| |
Reported by Jules Levin
ICAAC Sept 11-15 2009 San Francisco
A. SATO 1, T. SEKI 1, M. KOBAYASHI 1, T. YOSHINAGA 1, T. FUJIWARA 1, M. UNDERWOOD 2, E. GARVEY 2, B. JOHNS 2
1 Shionogi & Co., Ltd, Osaka, Japan, 2 GlaxoSmithKline, RTP, NC
ABSTRACT
Background: S/GSK1349572 is a potent once daily unboosted INI under clinical
development. It has demonstrated limited cross resistance to known INIs in vitro. We report here passage results from wild-type HIV and from INI-resistant single mutant clones.
Methods: HIV-1 NL432 and its single mutants E92Q, Q148H/K/R, and N155H, were passaged in MT-2 cells under increasing compound concentrations for 56 days; the IN gene was subsequently sequenced. Fold change (FC) of isolated mutants was determined with a HeLa-CD4 cell-line assay.
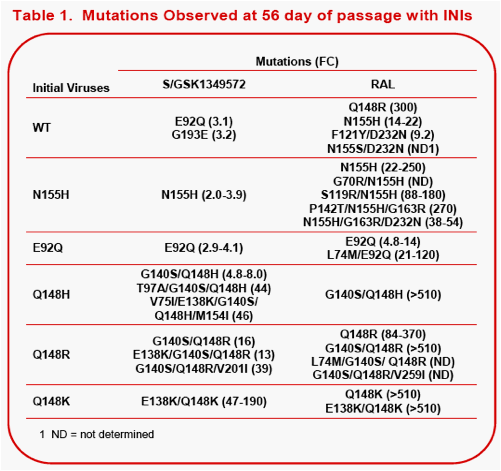
Results: Summary of representative INI-mutant genotypes (FC) on day 56.
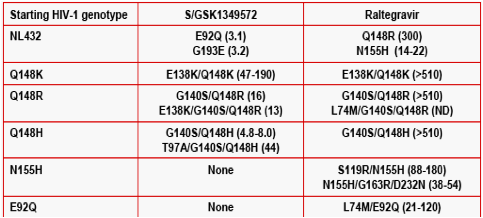
During passage studies with S/GSK1349572, an initial concentration of 32 nM prevented all virus replication. Virus with IN mutations selected in the presence of 6.4 nM S/GSK1349572 were unable to replicate at 160 nM. An initial concentration of 32 nM raltegravir allowed virus replication, and increasing concentration from 160 nM to 4,000 nM during passage selected single, double and triple mutants with FC 9.2 to >510.
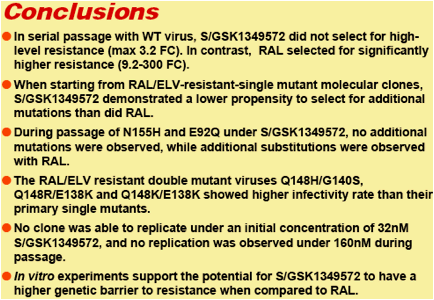
Conclusions: In serial passage with WT virus, S/GSK1349572 did not select for high-level resistance (max 3.2 FC); in contrast, RAL selected for significantly higher resistance (9.2-300 FC). When starting with HIV-1 with IN mutations, S/GSK1349572 demonstrated a lower propensity to select for additional mutations than did raltegravir. Together, these data suggest that S/GSK1349572 may have a higher genetic barrier to resistance than RAL.

Results & Discussion
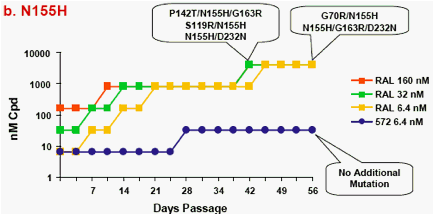
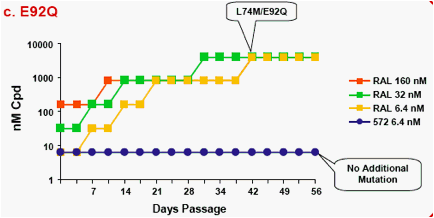
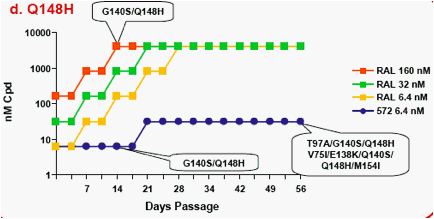
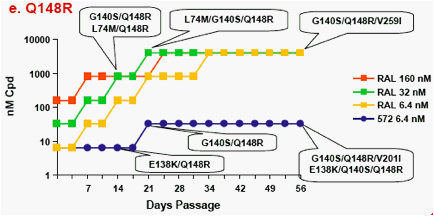
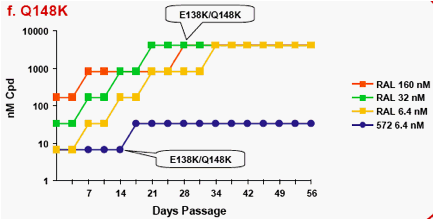
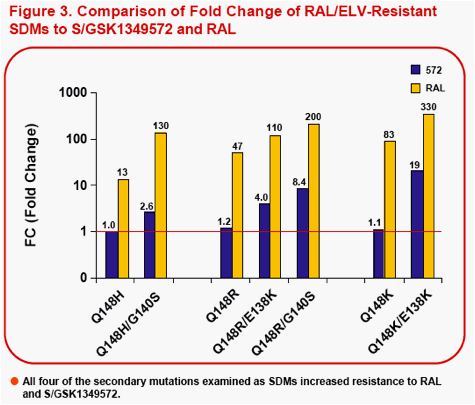
Figure 1a-f. Time Course of Emergence of Drug Resistant Viruses from Wild-type and RAL/ELV-resistant-single mutants at Indicated Compound Concentrations
- Dots and boxes indicate clear viral replication was observed microscopically, and each upside step indicates virus replicated when compound concentration was increased 5-fold.
- Viruses with RAL-resistant mutation(s) replicated in the presence of RAL at 4,000nM from day 14 - 28 of passage.
- Virus was unable to replicate under initial concentration of 32nM
S/GSK1349572, and during passage selected virus never replicated under >32nM S/GSK1349572.


REFERENCES
1. Lalezani J et al. IAS 2009, Cape Town, Abstract TUAB105.
2. Underwood M et al. IAS 2009, Cape Town, Abstract WEPEA098.
3. Song I et al. IAS 2009, Cape Town, Abstract WEPEB250.
4. Sato A et al. IAS 2009, Cape Town, Abstract WEPEA097.
5. Garvey EP et al. AAC. 2008; 52: 901-08
6. Kobayashi M et al. Antiviral Research. 2008; 80: 213-22.
7. Nakahara K et al. Antiviral Research. 2008; 81: 141-46
|
| |
|
 |
 |
|
|