 |
 |
 |
| |
Meta-Analysis of Safety for Short-term Dosing of an HIV Integrase Inhibitor, S/GSK1349572, from Seven Clinical Studies
|
| |
| |
Reported by Jules Levin
ICAAC Sept 11-15 2009
Y. LOU, S. MIN, S. CHEN, I. SONG, J. BORLAND, T. FUJIWARA, S. PISCITELLI
GlaxoSmithKline, RTP, NC, USA and Shionogi & Co., Ltd., Osaka, Japan

ABSTRACT
Background: S/GSK1349572 is an unboosted, once daily integrase inhibitor with a novel resistance profile that is currently in Phase 2 trials. Short-term safety was
evaluated from the first seven completed clinical studies, which included first time
in human, drug interaction, and Phase IIa studies in healthy and HIV-infected
integrase na´ve subjects.
Methods: S/GSK1349572 at doses between 2-100 mg administered in single or
repeat doses or placebo (PBO) was administered for up to 19 days. Adverse
events (AEs), labs, and ECGs were summarized by dose and by population
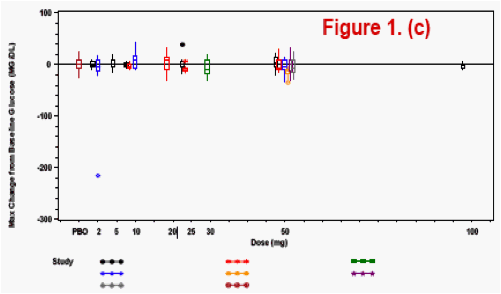
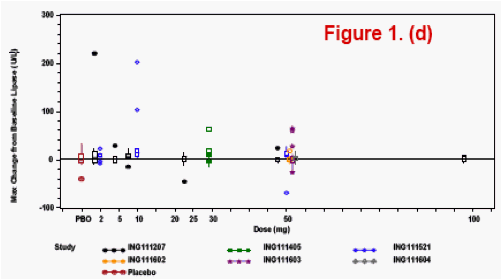
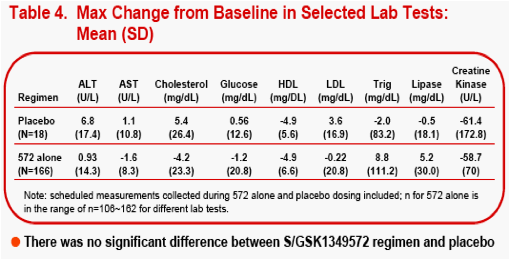
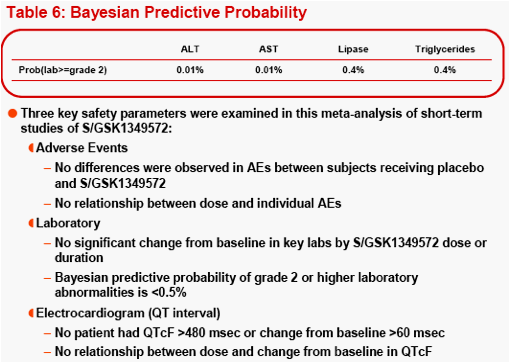
(healthy or HIV-infected subjects). Bayesian predictive probability of a subject with a Grade 2 or higher [PROB(Lab>=Grade2)] lab abnormality was calculated for triglyceride, lipase, ALT, and AST.
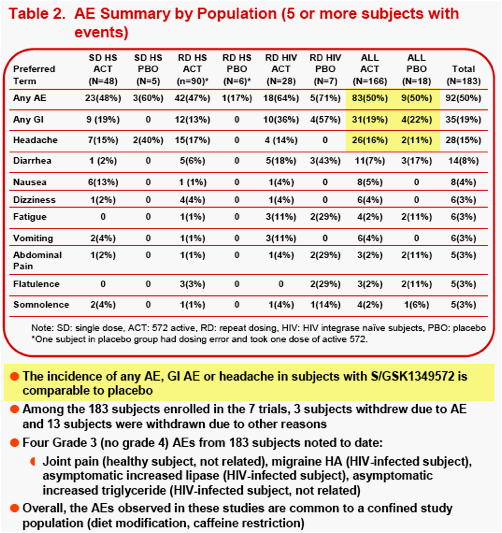
Results: 166 subjects received at least one dose of S/GSK1349572, and 17 subjects received PBO (total 183 enrolled). Three of 16 subjects withdrawn prematurely were due to AEs.
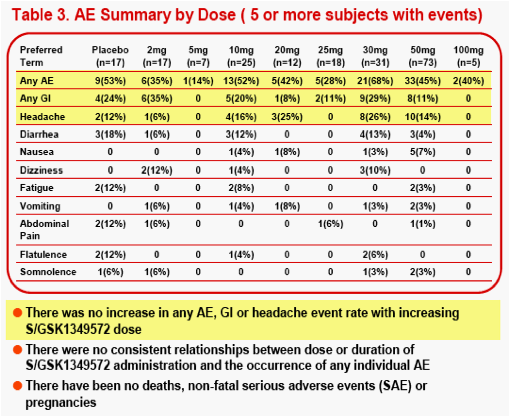
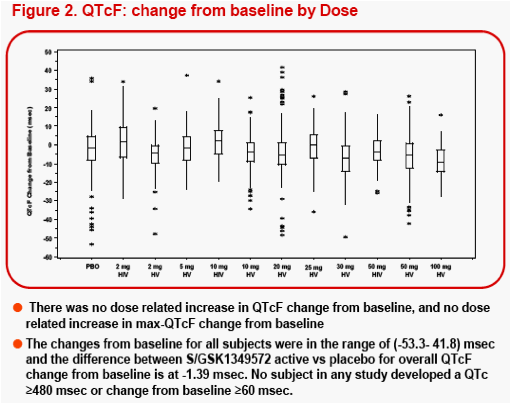
The most frequent AEs (≥6 subjects) observed during S/GSK1349572 dosing were headache (16%), diarrhea (7%), nausea (5%), dizziness (4%) and vomiting (4%). 3000 machine-read ECGs from 183 subjects were evaluated, and the difference in the mean change in QTcF from baseline between S/GSK1349572 and PBO groups, was -1.39msec.
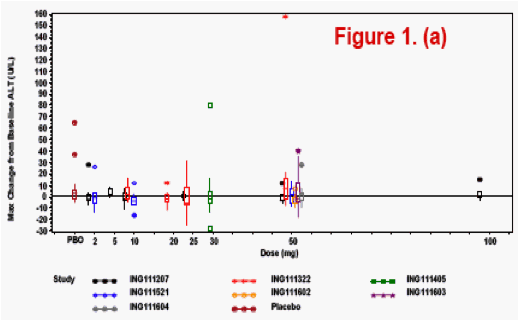
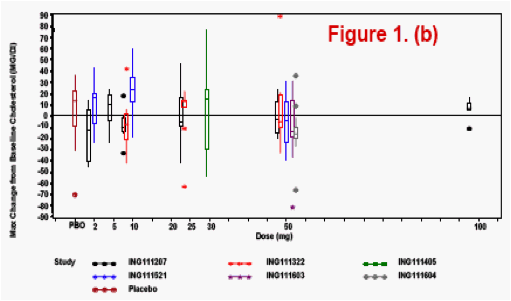
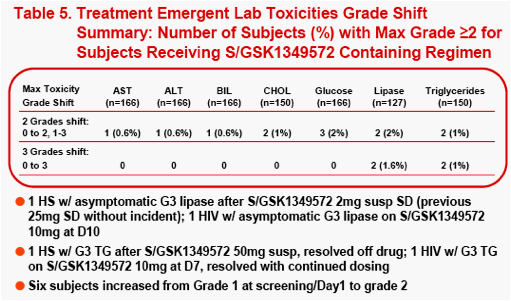
The most frequently observed grade 2 or higher lab abnormality was in total cholesterol, though total cholesterol elevations were frequently observed at baseline. No relationship was observed between dose level or dosing duration and AE rate, ECG change from baseline or lab test toxicities. The PROB(Lab>=Grade 2) was <0.5%.
Conclusion: S/GSK1349572 at single and repeat doses up to 100mg in healthy and HIV-infected subjects was well tolerated; the probability of moderate to severe laboratory abnormalities is predicted to be very rare. No clinically significant safety signals have been identified to date after short-term dosing with
S/GSK1349572.
INTRODUCTION
S/GSK1349572 is a once daily, unboosted integrase inhibitor (INI) which has
demonstrated unprecedented antiviral activity and good tolerability in short-term
clinical studies1,2
S/GSK1349572 is currently in Phase 2b clinical trials in both treatment-naive and
INI-resistant, HIV-infected patients
This meta-analysis was conducted to evaluate the overall short-term safety
profile of S/GSK1349572 (572)
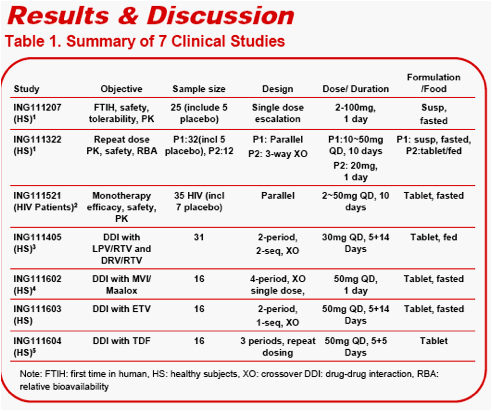
METHODS
183 subjects (S/GSK1349572: n=166, PBO: n=17) from six healthy subject trials
and one HIV subject trial were included the meta analysis
The following safety data were selected and combined : exposure, dropouts, AE,
ECG, lab
The following factors were considered in analysis: dose level, dose duration,
study
AE events were summarized by S/GSK1349572 dose level, by population (healthy vs HIV-infected) and dosing schedule (single vs repeat)
3000 ECGs were collected from 166 subjects who received at least one dose of
S/GSK1349572 and 17 placebo subjects. ECGs were collected as 2-3 pre-dose
measurements or single post-dose measurement at each timepoint, and summarized by S/GSK1349572 dose, and changes in QTcF for each subject were summarized and plotted
Changes from baseline and treatment emergent lab toxicities grades (DAIDS
scale) for selected lab tests were analyzed and plotted by dose or dosing duration
Lab toxicity risk was assessed by the Bayesian predictive probability for the selected lab tests, where non-informative prior distribution of Normal (0, 1000) was set for the variability of the lab tests.

References
1. Min S, et al. IAS 2009, Cape Town, abstract WEPEA099.
2. Lalezari J, et al. IAS 2009, Cape Town, abstract TUAB105.
3. Piscitelli S, et al. ICAAC 2009, San Francisco, Monday, abstract A1-1304, poster board 37.
4. Song I, et al. ICAAC 2009, San Francisco, Monday, abstract A1-1305, poster board 38.
5. Song I, et al. ICAAC 2009, San Francisco, Monday, abstract A1-1303, poster board 36.
|
| |
|
 |
 |
|
|