 |
 |
 |
| |
Emergence of mutations and phenotypic resistance with tipranavir treatment in protease inhibitor experienced patients
|
| |
| |
Reported by Jules Levin
18th Intl HIV Drug Resistance Workshop
June 9-12 Sunny Ft Myers Florida
David Hall1, Jonathan Schapiro2, John Baxter3, Charles Boucher4, Joseph Scherer5, MyriamWitvrouw6, Clemens Tilke5, Richard Bethell6
1Boehringer Ingelheim Pharmaceuticals,Connecticut,USA; 2National Hemophilia Center,Tel-Hashomer, Israel; 3Cooper University Hospital/UMDNJ-Robert Wood Johnson Medical School,New Jersey,USA; 4Erasmus Medical Center,Rotterdam, the Netherlands;
5Boehringer Ingelheim Germany, Ingelheim,Germany; 6Boehringer Ingelheim (Canada) Ltd, Laval,Québec,Canada
AUTHOR DISCUSSION
Virologic failure while on a tipranavir-based regimen does not lead to a decrease in median phenotypic susceptibility to DRV, APV, LPV or SQV, even in patients
who continue on a failing regimen.
There are several different patterns of selection related to tipranavir exposure. The most commonly selected mutations in patients who failed on a TPV-based
regimen were A82T, I84V, and I24L/M. The relationship to resistance and
cross-resistance will require further investigation.
L24M has not been reported previously as selected by PI-based therapy.With
tipranavir this represents a selection against L24I, associated with increased
susceptibility. Its impact on susceptibility for other protease inhibitors and paths
to resistance for other protease inhibitors remains to be explored.
ABSTRACT
Background: Virologic failure associated with the emergence of resistance to tipranavir (TPV) in patients receiving TPV-based therapy has been evaluated and reported in a small number of early failures. An investigation of all virologic failures in the RESIST trials provides a critical mass of new data for characterizing the effects of TPV selection for mutations in vivo.
Methods: TPV-treated patients who failed virologically in RESIST were identified. Baseline specimens, initial failure specimens, and later specimens when patients continued to take TPV were submitted for genotypic and phenotypic testing at Monogram Biosciences. Baseline and first failure results were compared to evaluate the selected mutation patterns and the patterns of protease inhibitor (PI) resistance.
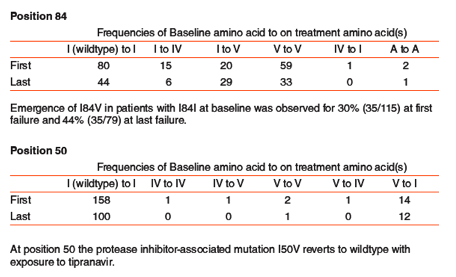
Results: Initial failure on TPV was associated with a 3.8 fold increase in Fold Change from baseline and continued treatment was associated with an additional 2.7 fold increase from initial to last failure observation. By contrast FC for DRV, FPV, LPV and SQV at initial TPV failure decreased by 10%, 20%, 30%, and 10%, respectively. At last failure FC for DRV had returned to above baseline while the others were unchanged. The most common emergent mutations at first failure were V82T, which emerged primarily in patients with the PI mutation
V82A at baseline (N=45), I84V, which emerged in patients with virus without a substitution at codon 84 at baseline (N=35), and L24L or M, which emerged in patients with the PI mutation L24I at baseline (N=23).
Conclusions: Virologic failure while on a tipranavir-based regimen does not lead to a change in phenotypic susceptibility to DRV, FPV, LPV or SQV, even in patients who continued on the failing regimen. The most commonly selected mutations in patients who failed on a TPV-based regimen were A82T, I84V, and I24L/M. L24M has not been reported previously as selected by PI-based therapy.
INTRODUCTION
Tipranavir is indicated for the treatment of protease inhibitor-experienced HIV patients. Phenotypic testing allows evaluation of the patterns of resistance to the different protease inhibitors and how the patterns are altered when a tipranavir-based regimen fails. Virologic failure associated with the emergence of resistance to tipranavir (TPV) in patients receiving TPV-based therapy has been evaluated and reported in a small number of early failures. An investigation of all virologic failures in the RESIST trials provides a critical mass of new data for characterizing the effects of TPV selection for mutations in vivo..
METHODS
TPV-treated patients who failed virologically in RESIST were identified.
Baseline specimens, initial failure specimens, and later specimens when patients continued to take TPV in spite of detectable viral replication were submitted for genotypic and phenotypic testing at Monogram Biosciences.
Baseline and first failure results were compared to evaluate the selected and deselected substitution patterns and the patterns of phenotypic susceptibility to other protease inhibitors (PIs).
Baseline and last failure results were compared to evaluate the effect of continued exposure to TPV after virologic failure on viral genotype and phenotype.
Change in phenotypic resistance was summarized by taking the ratio of fold changes and by performing a 2-sided paired t-test on the fold changes (Figure 1).
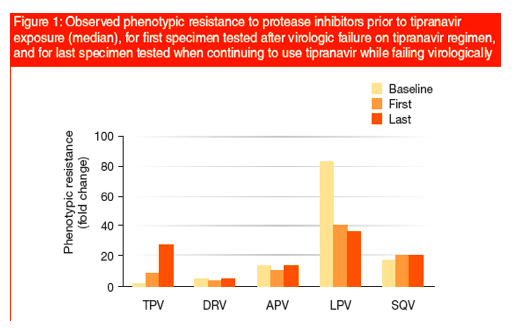
The median time to first failure was 16 weeks; to last failure was 72 weeks; time from first to last was 48 weeks.
Baseline resistance in both responders and non-responders was consistent with the history of exposure to 1st generation protease inhibitors, amprenavir (APV), lopinavir (LPV), and saquinavir (SQV).
Resistance to tipranavir increased with exposure to tipranavir and increased further with continued exposure while failing virologically.
Resistance to the other protease inhibitors tested remained stable or declined as seen with LPV (Figure 2).
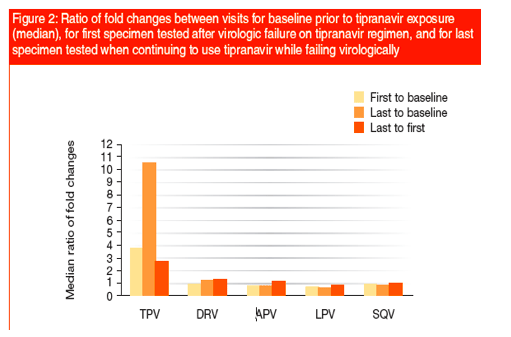
Ratios <1 indicate decreasing phenotypic resistance.While first failure specimens were consistently improving over baseline with respect to resistance to darunavir, amprenavir, lopinavir, and saquinavir, last failure specimens were regressing to greater resistance for darunavir and amprenavir (Table 1).
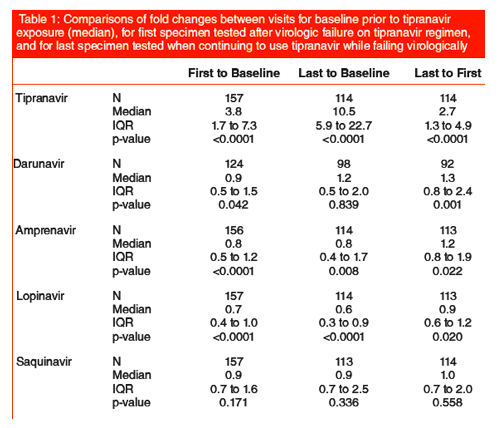
Tipranavir resistance continued to increase significantly with continued exposure, increasing at least 5-fold for 25% of patients who continued a failing tipranavir-based regimen.
Changes in darunavir resistance were highly variable, decreasing by a factor of 2 for 25% of patients while increasing by 50-100% for 25% of patients.
Amprenavir resistance decreased significantly from baseline at first failure and at last failure, while increasing significantly between first failure and last failure.
Lopinavir resistance decreased significantly for all comparisons, with 75% of patients having reduced resistance. The median reduction from baseline is 40% at last failure.
Saquinavir resistance was not significantly affected by tipranavir exposure.
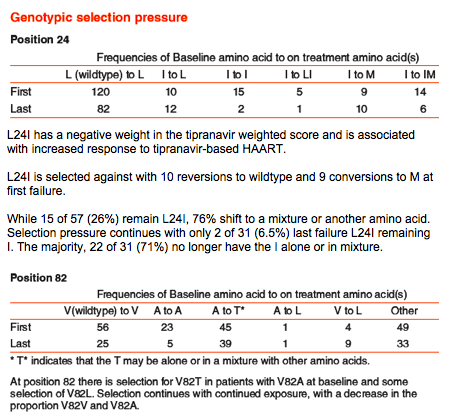
L24I has a negative weight in the tipranavir weighted score and is associated with increased response to tipranavir-based HAART.
L24I is selected against with 10 reversions to wildtype and 9 conversions to M at first failure.
While 15 of 57 (26%) remain L24I, 76% shift to a mixture or another amino acid.
Selection pressure continues with only 2 of 31 (6.5%) last failure L24I remaining I. The majority, 22 of 31 (71%) no longer have the I alone or in mixture.
Acknowledgement
Supported by Boehringer Ingelheim.
|
| |
|
 |
 |
|
|