 |
 |
 |
| |
Effectiveness and safety of Tenofovir disoproxil fumarate in field practice: a multicenter European cohort study of 737 patients with chronic hepatitis B
|
| |
| |
Reported by Jules Levin
AASLD Nov 2 2010 Boston
Pietro Lampertico1, Mauro Vigano1, Cihan Yurdaydin2, Ramazan Idilman2, Maria Buti3, Rafael Esteban3, George V. Papatheodoridis4, Giovambattista Pinzello5, Maria Vinci5, Eliseo Minola6, Fredy Suter16, Paolo Del Poggio7, Marco Andreoletti8, Stefano Fagiuoli9, Alberto Eraldo Colombo10, Andrea Salmi11, Teresa Santantonio12, Carlo Magni13, Guido Gubertini13, Francesco Fumagalli Maldini14, Natalia Terreni15, Floriana Facchetti1, Roberta Soffredini1, Massimo Colombo1
1) 1st Division of Gastroenterology, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Universita degli Studi di Milano, Milano, Italy. 2) Gastroenterology Division, University of Ankara Medical School, Ankara, Turkey. 3) Hospital General Universitario Valle Hberon and Ciberehd , Barcelona, Spain. 4) 2nd Dept. of Internal Medicine, Hippokration Hospital, Athens, Athens, Greece. 5) SC Epatologia e Gastroenterologia, Ospedale Niguarda Ca Granda, Milano, Italy. 6) Servizio Malattie Epatiche e Infettive, Humanitas Gavazzeni, Bergamo, Italy. 7) UO Epatologia, Ospedale di Treviglio, Treviglio, Italy. 8) S.C. Medicina Generale, Ospedale "A.Manzoni", Lecco, Italy. 9) Gastroenterology Unit, Liver and Lung Transplantation Center, Ospedali Riuniti di Bergamo, Bergamo, Italy. 10) Unita operativa di Medicina, Servizio di Epatologia, Ospedale Sant' Anna, Como, Italy. 11) Unita Operativa di Gastroenterologia, Fondazione Poliambulanza, Ospedale S.Orsola, Brescia, Italy. 12) Clinic of Infectious Diseases, Universita di Foggia, Foggia, Italy. 13) I and II Div. Infectious Diseases, Ospedale Luigi Sacco, Milano, Italy.14) Liver Center, Clinica Medica, Azienda Ospedaliera S. Gerardo, Universita Milano Bicocca, Monza, Italy. 15) UO Gastroenterologia, Ospedale Valduce, Como, Italy. 16) Infectious Diseases, Ospedali Riuniti di Bergamo, Begamo, Italy
ABSTRACT
Background and Aim. Registration studies showed Tenofovir disoproxil fumarate (TDF) to be an effective therapy for both NUC naïve and NUC resistant patients with chronic hepatitis B, but its effectiveness and safety in field practice is only partially established.
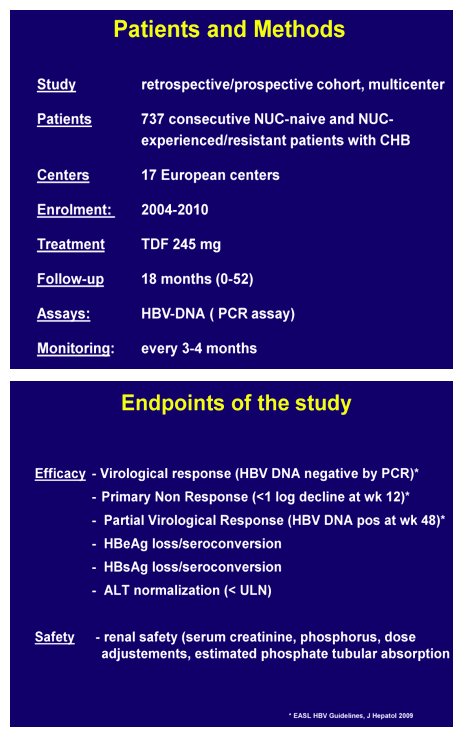
Methods: 737 patients on either mono or combo therapy with TDF were enrolled in a retrospective/prospective cohort study conducted in 17 Centers. Included were all patients with chronic hepatitis B starting TDF in each center, with the exclusion of HIV co-infected ones. Median follow-up was 16 months (range 0-52). Virological response was defined as undetectable HBV DNA by sensitive assays; safety analysis focused on renal and tubular function.
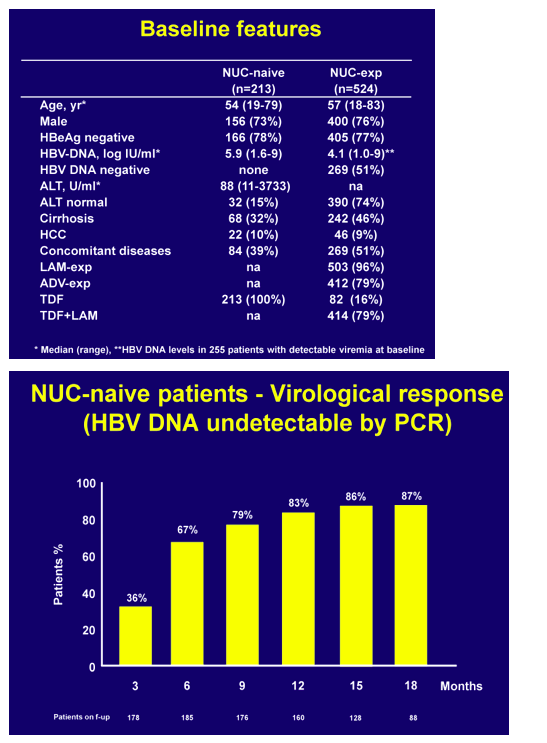
Results. Baseline features were: median age 56 years, 75% males, 77% HBeAg negative, 42% with cirrhosis, 9% with HCC, 30% with previous IFN treatment, 47 % with concomitant disease/medications, 71% NUC experienced or resistant and 29% NUC-naïve patients. TDF was given as a monotherapy in 27% of the patients and in combination, with lamivudine in most of the cases, in 72%. 14% of the patients started on reduced TDF dose because of reduced creatinine clearance.
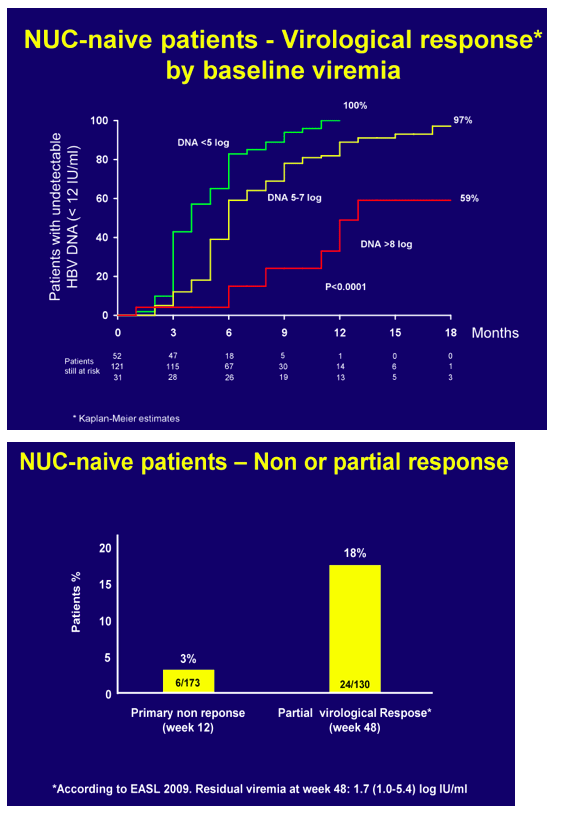
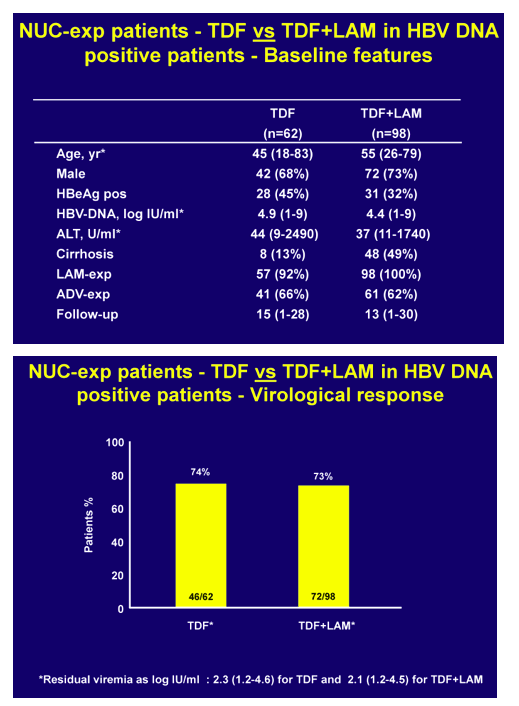
In NUC naïve patients, with a median baseline viremia of 5.9 log IU/ml (1.6 - >9), virological response rates were 37%, 66%, 72% and 89% at 12, 24, 36, 48 weeks with time to PCR negativity being significantly affected by baseline viral load.
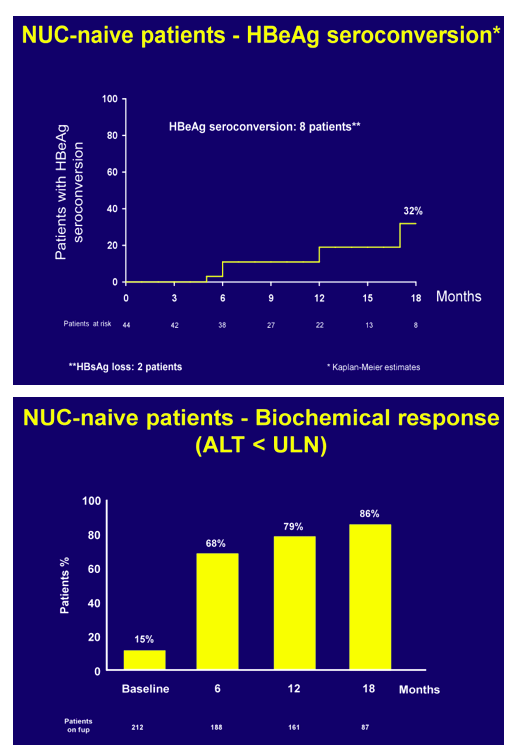
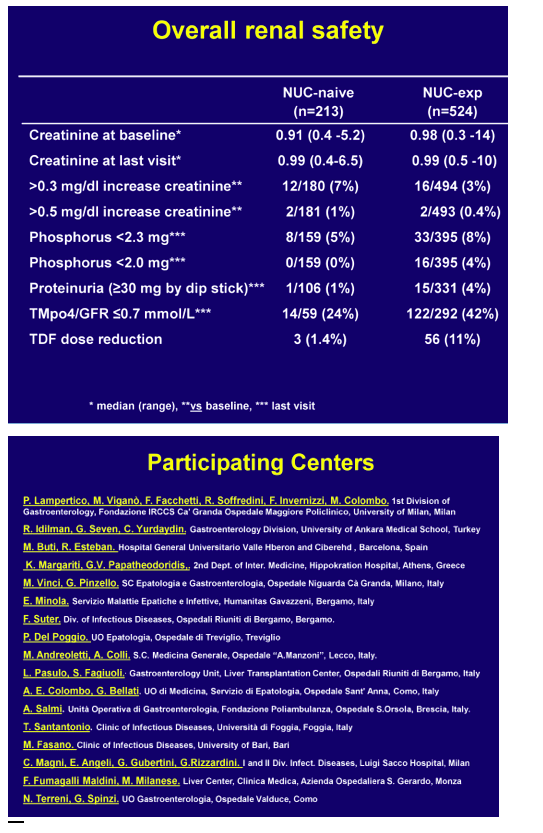
At 48 weeks, 17 patients had a partial virological response with a median 2.5 log IU/ml (1.3-5.4) residual viremia. In NUC-experienced or resistant patients, viremia remained undetectable in those who switched from ADV to TDF whereas it became undetectable in 74% of those with baseline ongoing HBV replication (median HBV DNA 4.1 log IU/ml, range 1->9 log), independently of the treatment strategy. In pooled safety assessment, a greater than 0.3 or 0.5 mg/dl increase of serum creatinine in the last visit versus baseline occurred in 3% and <1% of the patients, respectively. Blood phosphorus levels dropped below 2.3 mg/dl in 8% of the patients and urinary phosphate absorption, as assessed by estimated TmPI/GFR, decreased below 0.7 mmol/L in 37%: most of these patients had long-term previous exposure to ADV. Overall, TDF dose was reduced in 6% of the patients because of worsened creatinine clearance: most of these patients were older than 60 years, with previous exposure to ADV and with concomitant potentially nephrotoxic diseases/medications.
Conclusions. TDF suppressed HBV replication in most NUC-naïve and NUC-experienced or resistant patients in field practice. Few patients showed an impaired renal and tubular function of multifactorial origin.

|
| |
|
 |
 |
|
|