 |
 |
 |
| |
Efficacy and Safety of Entecavir Versus Adefovir in Chronic Hepatitis B Patients with Evidence of Hepatic Decompensation
|
| |
| |
Reported by Jules Levin
20th Conference of the APASL, China National Convention Center, 25 - 28 March 2010, Beijing, China
Y.-F. Liaw1, M. Raptopoulou-Gigi2, H. Cheinquer3, S.K. Sarin4, T. Tanwandee5, N. Leung6, R.P. Myers7, R.S. Brown Jr8, M. Shiffman9, J. Bialkowska10, S. Tang11, E. Cooney11
1Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taipei, Taiwan; 2Department of Internal Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece; 3Universidade Federal Do Rio Grande Do Sul, Porto Alegre, Brazil; 4Department of Gastroenterology G B Pant Hospital and Institute of Liver and Biliary Sciences, New Delhi, India; 5Department of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; 6Alice Ho Miu Ling Nethersole Hospital, Hong Kong, China; 7Liver Unit, University of Calgary, Alberta, Canada; 8Center for Liver Disease and Transplantation, Columbia University Medical Center, New York, USA; 9McGuire VA Medical Center, Richmond, Virginia, USA; 10Department of Infectious Diseases, Medical University, Lodz, Poland; 11Research and Development, Bristol-Myers Squibb Company, Wallingford, USA
Introduction
Decompensated cirrhosis is one of the major sequelae of longstanding hepatitis B virus (hbv ) infection. At 5 years, survival of patients with decompensated cirrhosis was 14%, compared to 84% for patients with compensated cirrhosis1
Suppression of viral replication with antiviral therapy has been shown to result in clinical improvement and increased survival2,3
Interferons are contraindicated in this patient population4,5
Data on the safety and efficacy of nucleos(t)ide therapy in patients with chronic hepatitis B (chb ) and decompensated liver disease are limited
Entecavir (ETV) has demonstrated superior virologic, histologic and biochemical efficacy compared to lamivudine (LVD) in nucleosidena´ve patients with CHB and compensated liver disease at Week 486,7
Long-term ETV therapy resulted in durable suppression of viral replication and regression of fibrosis/cirrhosis in CHB patients with compensated liver disease8
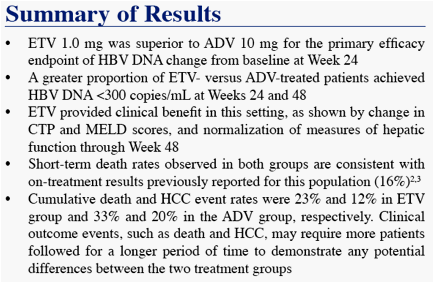
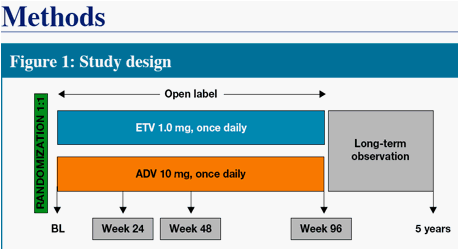
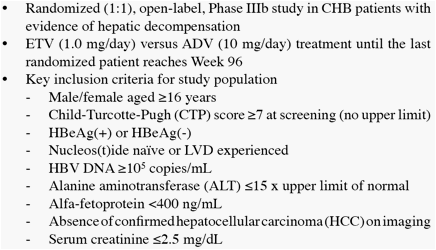
We present Week 48 results from a randomized, open-label, comparative study of ETV versus adefovir (ADV) in CHB patients with decompensated liver disease
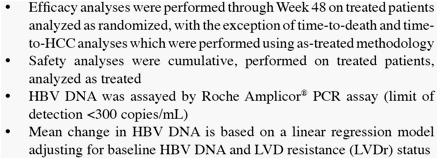
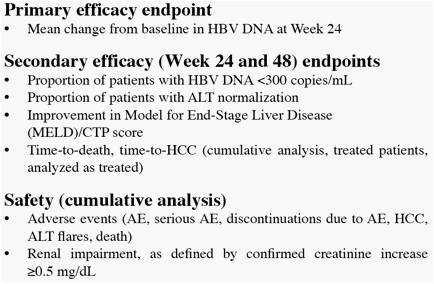

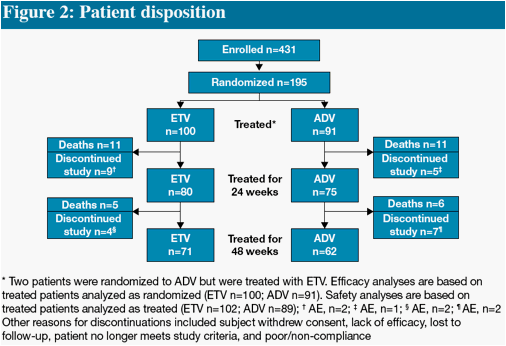

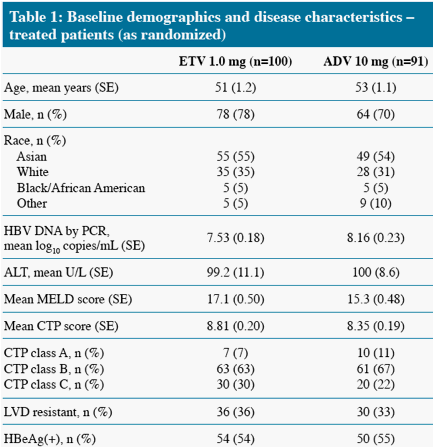
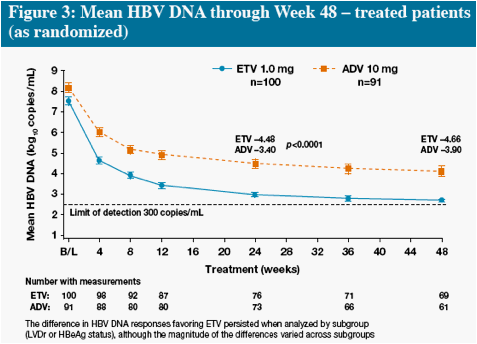
* The difference in HBV DNA responses favoring ETV persisted when analyzed by subgroup (LVDr or HBeAg
status), although the magnitude of the differences varied across subgroups
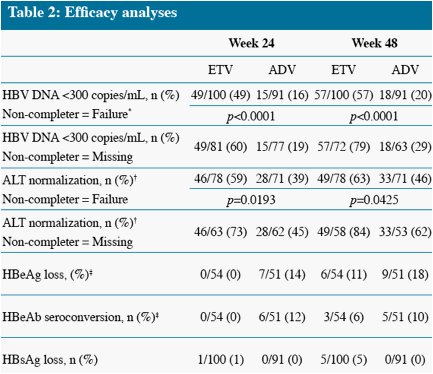
Analysis limited to patients with abnormal ALT at baseline
Analysis limited to HBeAg(+) patients at baseline, non-completer = Failure
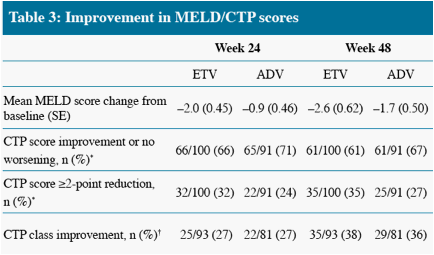
* Non-completer = Failure
CTP class C/B to class A only
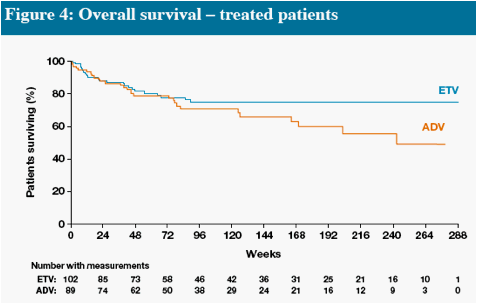
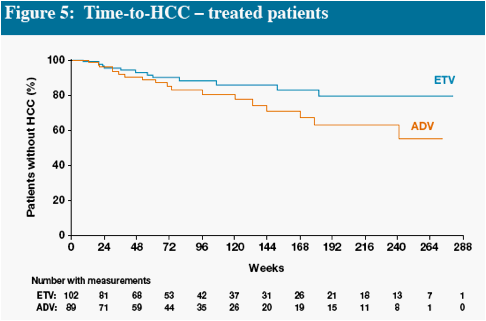
In Figures 4 and 5, interpretation of data beyond Week 48 is limited at the time of this will provide more robust estimates
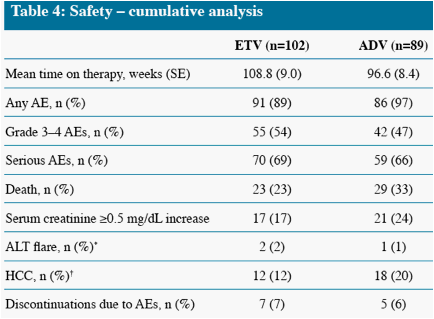
Death and HCC include events which occurred on and off treatment, all other parameters measure on-treatment events only
* One additional event occurred off treatment in the ADV group
Other remaining malignancies (both in ETV group) were: recurrent non-Hodgkin lymphoma (n=1), basal cell carcinoma (n=1)
REFERENCES
[1] Zoulim E, et al. Liver Transpl 2008;14:S1-S7. [2] Fontana R, et al. Gastroenterology 2002;123:719-27.
[3] Schiff E, et al. Liver Transpl 2007;13:349-60. [4] Lok ASF & McMahon BJ. Hepatology 2009;50:1-36.
[5] European Association for the Study of the Liver. J Hepatol 2008;50:227-42.
[6] Chang TT, et al. N Engl J Med
2006;354:1001-10.
[7] Lai C-L, et al. N Engl J Med 2006;354:1011-20.
[8] Liaw YF, et al. Hepatology 2009;48:706A.
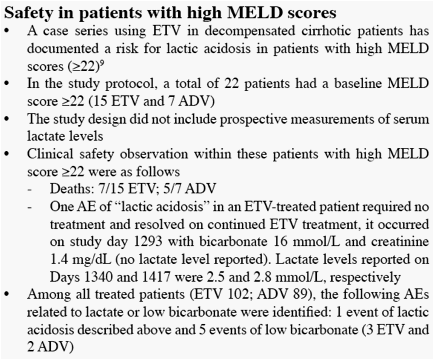
[9]
Sarrazin et al. Hepatology 2009;HEP-09-0827
|
| |
|
 |
 |
|
|