 |
 |
 |
| |
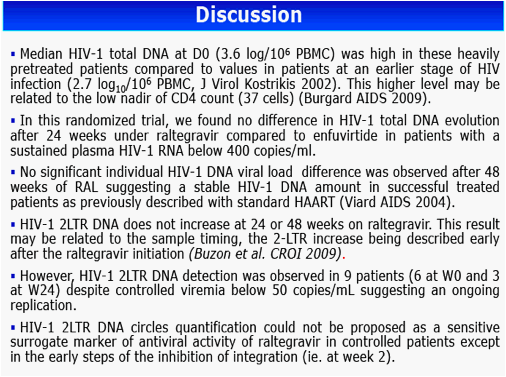
No Evolution of HIV-1 Total DNA and 2-LTR Circles after 48 Weeks of Raltegravir-containing Therapy in Patients with Controlled Viremia: A Sub-study of the Randomized EASIER-ANRS 138 Trial
|
| |
| |
Reported by Jules Levin
CROI 2010
Constance de Laugerre1, I Charreau2, J Braun2, M-L Nere1, N de Castro1, P Yeni3, J Ghosn4, F Simon1, J-P Aboulker2, and J-M Molina1
1Univ Paris 7 Diderot, Saint-Louis Hosp, AP-HP, France; 2INSERM SC10, Villejuif, France; 3Univ Paris 7 Diderot, Bichat-Claude Bernard Hosp, AP-HP, France; and 4Hosp Kremlin-Bicetre, AP-HP, France
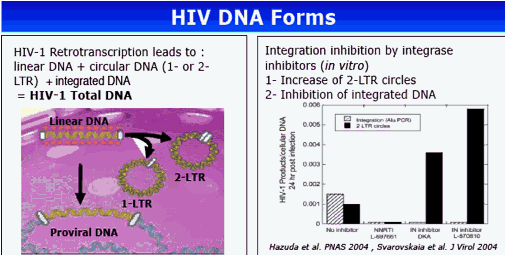
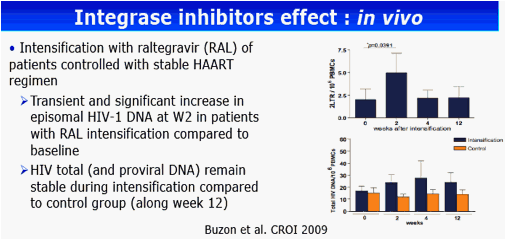
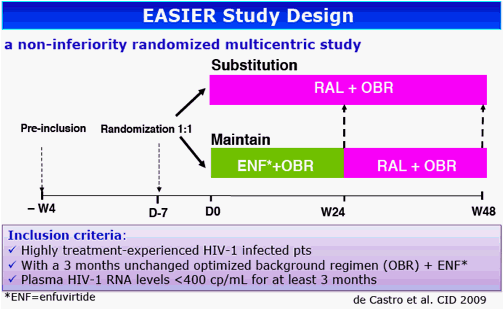
Background: Integrase inhibitors have induced in vitro a decrease of proviral DNA level in HIV-infected cells and conversely, an increase of the episomal viral DNA 2-LTR forms. The EASIER-ANRS 138 randomized trial demonstrated previously the virological non-inferiority of the switch from enfuvirtide (EFV) to raltegravir (RAL) in 169 highly treatment-experienced patients with plasma HIV-1 RNA (pVL) <400 copies/mL. The aim of this study was to analyze the effect of RAL on the evolution of HIV-1 total DNA and episomal cDNA forms in patients with a controlled viral load.
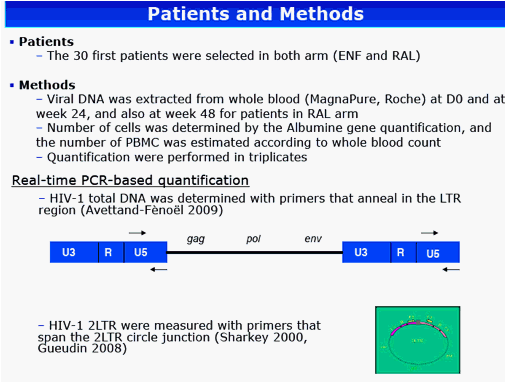
Methods: HIV DNA was extracted from whole blood and quantified in the first 30 patients from each arm at weeks 0 and 24 and also at week 48 for patients in the RAL arm. The 2-LTR circles DNA were quantified with primers spanning the 2-LTR circle junction and total HIV-1-DNA was assayed using internal LTR primers.
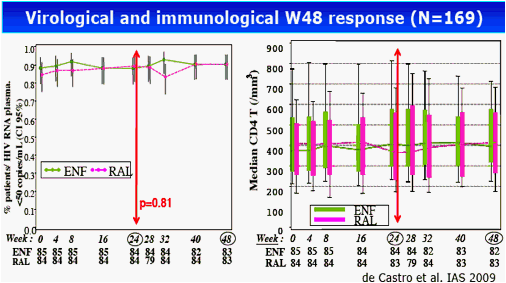
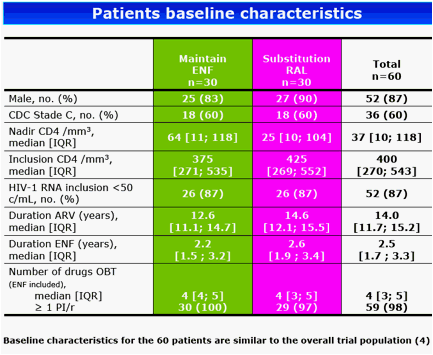
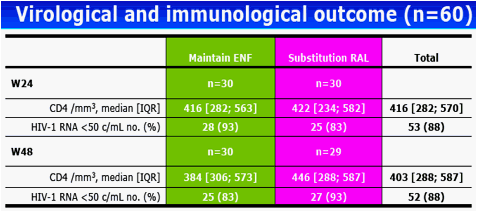
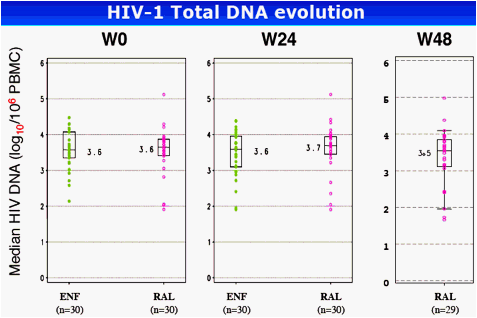
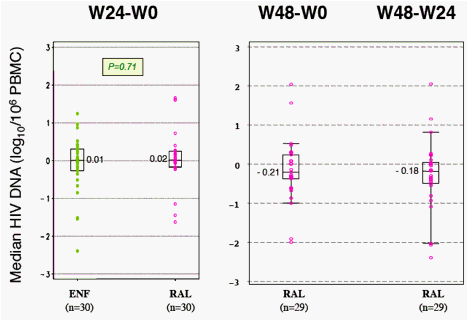
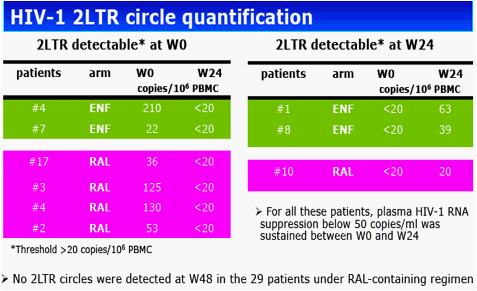
Results: The 60 patients were characterized at baseline as 60% CDC stage C; 14 years, median HAART duration; 2.5 years, median EFV duration; 87% (52 of 60) median baseline and nadir CD4 cell count of 400 cells/mm3 and 37 cells/mm3, respectively; and <50 copies/mL plasma viral load. At baseline, median total DNA was 3.61 log10/106 peripheral blood mononuclear cells (PBMC) and 2-LTR circles were detected in 6 patients with a median of 89 copies/106 PBMC. At week 24, CD4 cell count was 416 cells/mm3 and 88% of patients had a plasma viral load of <50 copies/mL. Total DNA was 3.68 log10/106 PBMC and 2-LTR circles were detected in 3 patients (with no 2-LTR circles at baseline). At week 48, among the 29 patients still receiving RAL, CD4 cell count was 446 cells/mm3, 93% of patients had a plasma viral load <50 copies/mL, total DNA was 3.54 log10/106 PBMC and no 2-LTR circles were detected. No significant change was observed between week 24 and week 0 in total DNA in both randomization groups: (0.01, -0.27 to 0.30, and 0.02, -0.17 to 0.25, log10 in the EFV and RAL groups, respectively, P = 0.71, Wilcoxon test). Also, no significant change was observed in the RAL arm between week 48 and week 0 (-0.2, -0.37 to 0.24) and between week 48 and week 24 (-0.18, -0.49 to 0.04).
Conclusions: In this randomized trial, no change in HIV total DNA was observed over 48 weeks in well-suppressed patients switching from EFV to RAL, suggesting that the majority of viral DNA was non-dynamic in those patients. Quantification of 2-LTR DNA after as long as 48 weeks of RAL did not seem to be a sensitive surrogate maker of integration inhibition.

|
| |
|
 |
 |
|
|