 |
 |
 |
| |
Combination of TMC435 with two novel NS5B inhibitors increases anti-HCV activity and results in a higher genetic barrier in vitro
|
| |
| |
Reported by Jules Levin
45th Annual Meeting of the European Association for the Study of the Liver (EASL), Vienna, Austria, 14-18 April, 2010
O. Lenz, J.M. Berke, H. de Kock, P. Van Remoortere, O. Nyanguile, S. Vendeville, T. Jonckers, P. Raboisson, K. Simmen, G. Fanning, T. Lin
Tibotec BVBA, Belgium
AUTHOR CONCLUSIONS
The in vitro replicon studies reported here show that combined treatment with an HCV NS3/4A protease inhibitor (TMC435) and an HCV NS5B polymerase NNI or NI:
- is additive or synergistic, with no antagonism observed
- increases anti-HCV activity and raises the genetic barrier to resistance
- results in improved clearance of replicon HCV RNA.
In vitro treatment with a combination of all three inhibitors at low concentration further increases replicon HCV RNA clearance.
These in vitro virology findings support the further evaluation of TMC435 in combination with HCV NS5B inhibitors.
Introduction
TMC435 is a macrocyclic NS3/4A protease inhibitor currently in Phase IIb clinical development for the treatment of hepatitis C virus (HCV) infection.
It is a potent and selective inhibitor of NS3/4A in vitro, with a 50% effective concentration (EC50) of 8 nM in a genotype-1b replicon cell line.1
Findings from Phase I and IIa studies have demonstrated that TMC435 is well tolerated, has a pharmacokinetic profile that supports a once-daily (QD) dosing regimen, and demonstrates potent antiviral activity in both treatment-naive and -experienced genotype-1-infected patients.2-5
Since combinations of specifically targeted antiviral therapies for HCV (STAT-Cs) with different mechanisms of action may provide more efficacious HCV treatment, we performed in vitro replicon studies to assess the potential of combining TMC435 with one or two novel HCV NS5B polymerase inhibitors (a nonnucleoside inhibitor [Tib-NNI] and a nucleoside inhibitor [Tib-NI]); here we report these findings.

RESULTS
Effect of inhibitor combinations in an antiviral assay
The effect of combining TMC435, Tib-NNI and Tib-NI on anti-HCV activity
is shown in Figure 1 and Table 1.
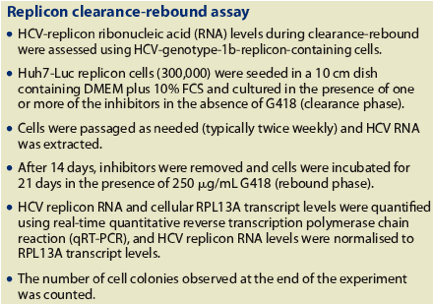
Figure 1. Effect of combining (A) TMC435 and Tib-NI, (B) TMC435 and a Tib-NNI, and (C) Tib-NI and Tib-NNI on antiviral activity
Three-dimensional synergy plots at the 95% confidence interval, as produced by
the MacSynergy II software for representative experiments are shown
CI, confidence interval
Table 1. Antiviral activity of different combinations of TMC435, Tib-NNI and Tib-NI.

Synergy and antagonism volumes at the 95% confidence interval (CI), as produced by the MacSynergy II software. Synergy volumes of <25, 25-50, 50-100 and >100 indicate insignificant synergism, slight synergism, moderate synergism and strong synergism, respectively. Results shown are averages from two or more experiments
· Treatment of the cells with TMC435 in combination with Tib-NNI or Tib-NI resulted in additive or synergistic anti-HCV activity, respectively.
· Treatment with Tib-NNI in combination with Tib-NI resulted in additive anti-HCV activity.
· No cytotoxicity was observed with any of the combinations tested.
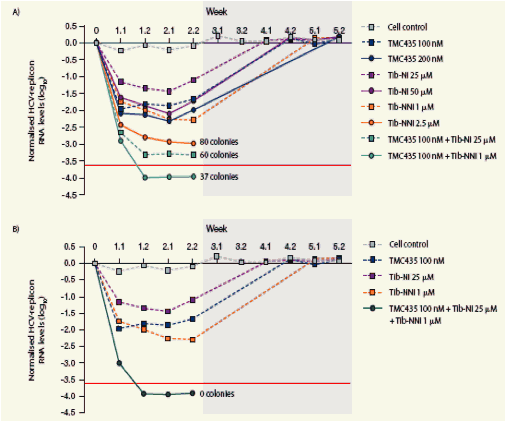
Effect of inhibitor combinations on colony formation
Cell colony formation, in the presence of TMC435, Tib-NNI or Tib-NI alone and in combination, is shown in Figure 2.
Treatment with TMC435 in combination with Tib-NNI or Tib-NI prevented the formation of resistant replicon colonies.
Treatment with Tib-NNI in combination with Tib-NI prevented the formation of resistant replicon colonies at the lowest concentration tested.
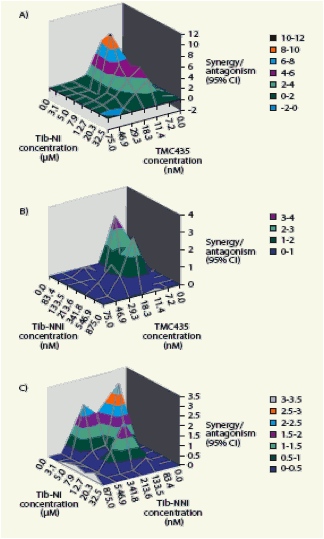
Replicon clearance-rebound assay
Clearance of HCV RNA from replicon-containing cells in the presence (clearance phase) and absence (rebound phase) of TMC435, Tib-NNI or
Tib-NI alone and in combination are shown in Figure 3.
All three inhibitors reduced replicon HCV RNA levels during the 2-week clearance phase, but did not lead to total clearance of replicon HCV RNA from cells.
Treatment with TMC435 in combination with Tib-NNI or Tib-NI increased the initial HCV RNA reduction, although a few replicon colonies were observed in the rebound phase, suggesting incomplete replicon HCV RNA clearance for some combinations.
Treatment with a combination of all three inhibitors at the lowest concentrations tested resulted in a pronounced reduction in replicon HCV RNA and the most efficient replicon clearance.
Figure 3. Clearance of HCV RNA from replicon-containing cells in the presence of TMC435, Tib-NI and Tib-NNI alone and in combination.
The rebound phase is shaded in grey, and the RT-PCR cut-off is shown as a red line. The number of surviving replicon cell colonies is indicated.
HCV, hepatitis C virus; RNA, ribonucleic acid; RT-PCR, reverse transcription polymerase chain reaction
Figure 2. Cell colony formation in the presence of TMC435, Tib-NI or Tib-NNI alone (A), or in combination (B and C).
The number of surviving cell colonies is indicated on the right lower corner
for each cell culture dish EC50, 50% effective concentration
· Increasing concentrations of each inhibitor alone resulted in a dose-dependent
reduction in colony formation but did not completely prevent resistant replicon colony formation
References
1. Lin TI et al. Antimicrob Agents Chemother 2009; 53: 1377-1385.
2. Reesink HW et al. Gastroenterology 2010; 138: 913-921.
3. Reesink HW et al. Poster presented at the 60th AASLD meeting, Boston, MA, USA, 30 October-3 November, 2009.
4. Marcellin P et al. Poster presented at the 44th Annual Meeting of European Association for the Study of the Liver (EASL), Copenhagen, Denmark, 22-26 April, 2009.
5. Manns M et al. Presented at the 44th Annual Meeting of European Association for the Study of the Liver (EASL), Copenhagen, Denmark, 22-26 April, 2009.
6. Pritchard MN, Shipman C Jr. Antiviral Res 1990; 14: 181-205.
|
| |
|
 |
 |
|
|