 |
 |
 |
| |
Low Rates of Nucleos(t)ide-associated Adverse Events
in the Long-term Experience with Entecavir
|
| |
| |
Reported by Jules Levin
Ar 14-18 Vienna Austria, 45th Annual Meeting of the European Association for Study of the Liver, 2010
M. Manns1, U.S. Akarca2, T.-T. Chang3, W. Sievert4, S.-K. Yoon5, N. Tsai6, A. Min7, A. Pangerl8, S. Beebe9, M. Yu9, S. Wongcharatrawee9
1Department of Gastroenterology, Hepatology, and Endocrinology, Hannover Medical School, Hannover, Germany; 2Department of Gastroenterology, Faculty of Medicine, Ege University, Izmir, Turkey; 3Department of Internal Medicine, National Cheng Kung University Medical College, Tainan, Taiwan; 4Department of Medicine, Monash University, Monash Medical Centre, Melbourne, Australia;
5Kangnam St. Mary Hospital, Catholic University of Korea, Seoul, Korea; 6John A. Burns School of Medicine, University of Hawaii, Honolulu, Hawaii; 7Beth Israel Medical Center, New York, USA; 8Research and Development, Bristol-Myers Squibb Company, Munich, Germany; 9Research and Development, Bristol-Myers Squibb Company, Wallingford, CT, USA
Background
Long-term nucleos(t)ide analogue (NA) therapy is recommended for chronic hepatitis B (CHB) patients with compensated and decompensated liver disease who cannot achieve a sustained virological response off-treatment, or those with advanced liver disease1,2
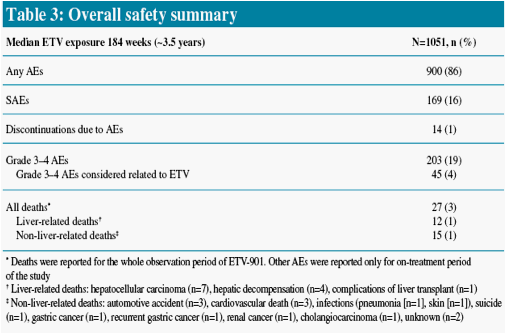
NAs approved for the treatment of CHB are generally well tolerated; however, some NA-specific adverse events (AEs) have been identified3
Long-term treatment with entecavir (ETV) results in durable viral suppression and is associated with regression of fibrosis/cirrhosis4-6
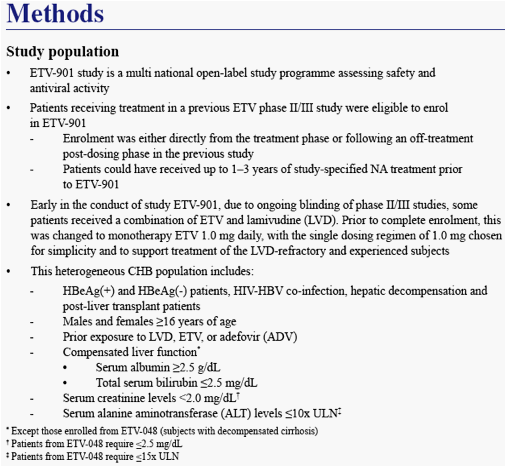
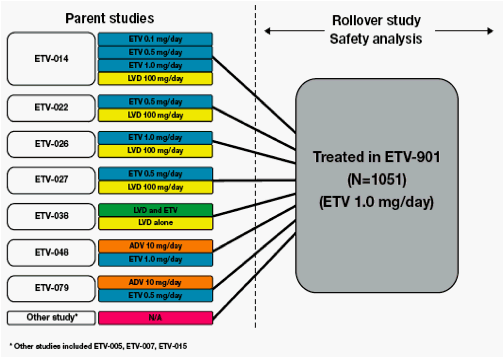
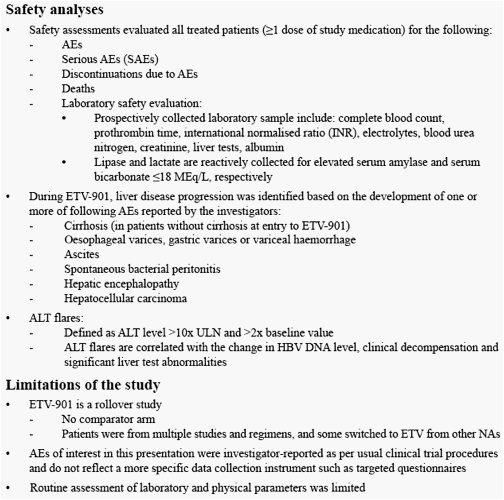
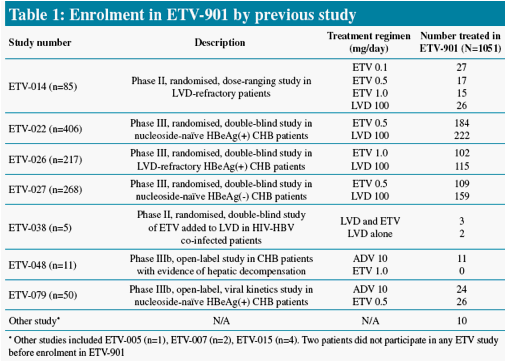
Patients who had completed treatment in one of the previous ETV phase II/III studies could enroll in the rollover study ETV-901
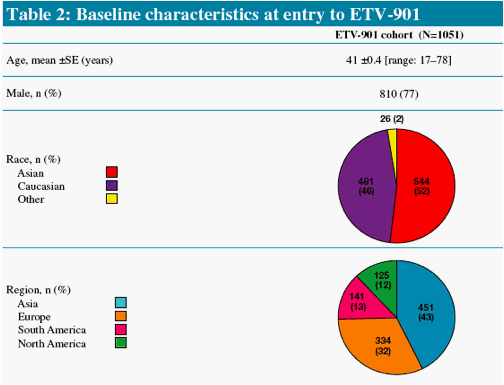
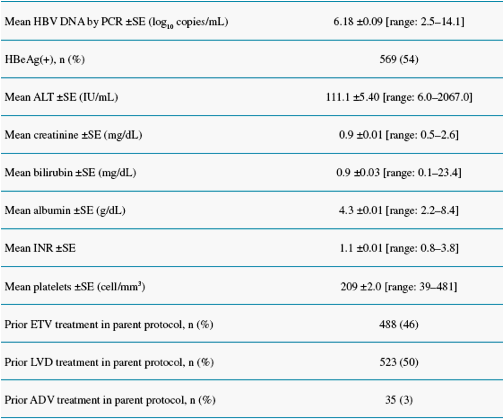
Study ETV-901 provides an opportunity to assess safety events in a large cohort of diverse CHB patients on ETV
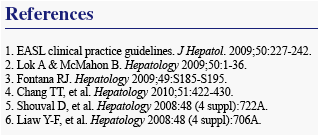
We present safety data from patients who received long-term ETV (1.0 mg/day) therapy in study ETV-901, focusing on AEs with potential NA association



|
| |
|
 |
 |
|
|