 |
 |
 |
| |
Preclinical characterization of non-covalent
HCV NS3/4A protease inhibitor BI 201335
|
| |
| |
Reported by Jules Levin
Apr 14-18 2010
45th EASL- Vienna Austria
Peter W. White, Montse Llinas-Brunet, Ma'an Amad, Richard C. Bethell, Gordon Bolger, Michael G. Cordingley, Jianmin Duan, Michel Garneau, Lisette Lagace, Diane Thibeault, George Kukolj
Boehringer Ingelheim (Canada) Ltd, Laval, Quebec, Canada
ABSTRACT
Background and Aims: Potent direct-acting antiviral agents that can be conveniently administered at relatively low doses are expected to improve treatment options for HCV-infected patients. BI 201335 is an inhibitor of HCV NS3/NS4A protease, which gave rise to maximal viral load reductions of up to 4.0 log10 when dosed at 240 mg once-daily as monotherapy to treatment-naïve patients for 14 days. Here we present the preclinical properties of this promising new antiviral agent.
Methods and Results: BI 201335 is a highly optimized non-covalent competitive inhibitor of full-length NS3/4A proteases of genotypes (GT) 1a and 1b with Ki values of 2.6 and 2.0 nM, respectively, and several thousand-fold selectivity relative to host cell proteases. Ki values of 2-250 nM were measured against the NS3/4A proteases of GT 2-6. The EC50 values of BI 201335 are 6.5 and 3.1 nM in GT 1a and 1b replicon assays, with an in vitro selectivity index of 4,600 for GT 1a. Combinations of BI 201335 with either interferon or ribavirin had additive effects in replicon assays. BI 201335 had good permeability in Caco-2 assays (8.7 and 8.1 x 10-6 cm/s in the apical-basolateral and basolateral-apical directions) and high metabolic stability after incubation with human, rat, monkey, and dog liver microsomes (half-life values of 190, 1,300, 91, and 470 minutes, respectively). Its favorable pharmacokinetic profile in rat, monkey, and dog predicted that therapeutic concentrations in man could be achieved at a moderate dose. Furthermore, drug levels were significantly higher in rat liver than in plasma at all timepoints measured (AUC 42-fold higher), suggesting that distribution to the target organ is especially favorable.
Conclusions: BI 201335 is a highly potent inhibitor of NS3 protease activity and HCV replication in biochemical and cellular assays. Its in vitro ADME properties and animal pharmacokinetics are consistent with its excellent human PK profile. Based on its high level of in vitro activity and selectivity, as well as promising initial clinical antiviral results achieved with a convenient dosing regimen, BI 201335 has been advanced to large phase IIb clinical trials in both treatment-naïve and treatment-experienced patients.
Introduction
BI 201335 is an inhibitor of hepatitis C virus (HCV) genotype 1 (GT 1) NS3/4A protease, which was selected for development based on its potent and selective activity and its very good in vitro and in vivo ADME-pharmacokinetic (PK) properties
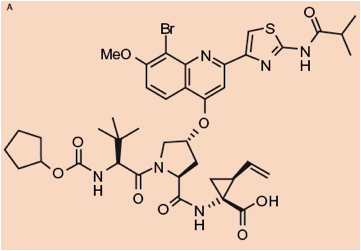
BI 201335 is structurally distinct from most other reported NS3/4A protease inhibitors currently in clinical development
- It is a linear tripeptide structure which makes optimized interactions with the NS3 protease substrate binding site
Here we present the full preclinical profile of this promising new direct-acting antiviral agent
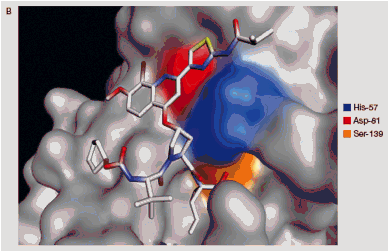
FIGURE1. A) Chemical structure of BI 201335; B) BI 201335 bound to the GT 1 NS3 protease domain (active site residues highlighted)
RESULTS

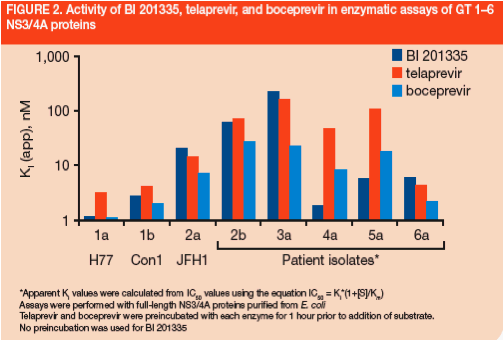
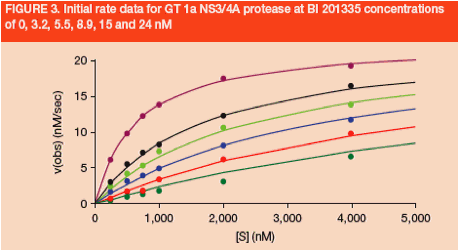
BI 201335 was shown to be a competitive inhibitor of GT 1a (Figure 3) and GT 1b NS3/4A proteases
-- Ki values from initial-rate kinetics were determined to be 2.6 and 2.0 nM for GT 1a and 1b enzymes, respectively
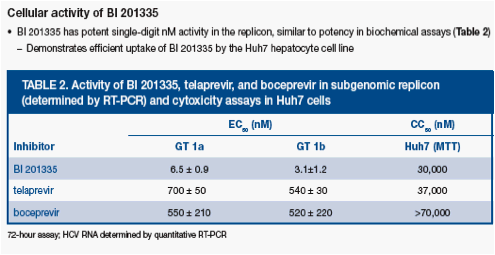
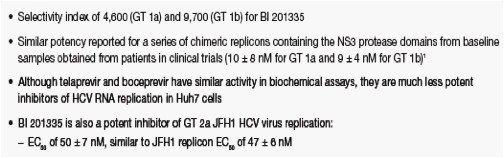
BI 201335 was found to be highly selective against human leukocyte elastase (HLE) and cathepsin B (CatB) (Table 1), and had no activity (>10 µM) against a panel of 39 other human proteases
- High selectivity compared to telaprevir and boceprevir is due to the unusual affinity of NS3 for C-terminal carboxylic acids

In vitro combination with interferon-a (IFN-a)
· As expected for a direct-acting antiviral, the combination of BI 201335 and IFN-a was additive (Figure 4)
· A similar conclusion was reached for the combination of BI 201335 with ribavirin, though data (not shown) are less complete due to the small window between anti-HCV activity and cytoxicity for ribavirin in cell culture
· The combination of BI 201335 with IFN-a was very effective at suppressing the emergence of resistance in the replicon system2
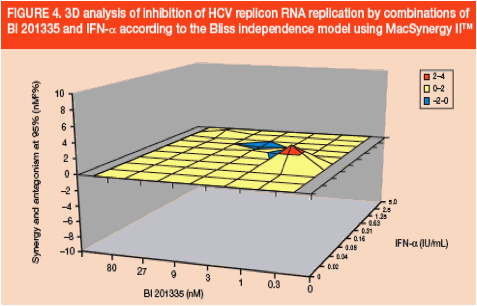
· The volume of synergy and antagonism at 95% confidence were 3.9 and -0.29 nM2% respectively, indicating that this combination is additive
· Similar results were obtained in three independent combination experiments
In vitro ADME properties
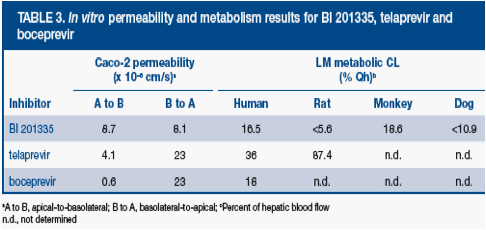
· BI 201335 demonstrated good Caco-2 permeability, with similar basolateral-to-apical permeability suggesting BI 201335 is not subject to extensive efflux (Table 3)
· Low in vitro Phase I liver microsome clearance in all species demonstrates that BI 201335 is not a good substrate for metabolism by Phase I enzymes, such as cytochrome P-450s
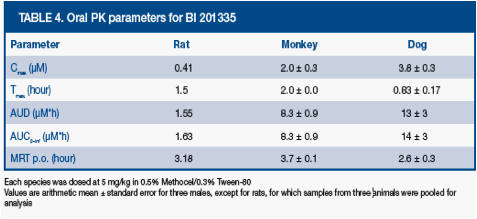
Cross-species PK
· BI 201335 was rapidly absorbed in three preclinical species (Table 4), with Cmax and AUC increasing with body mass
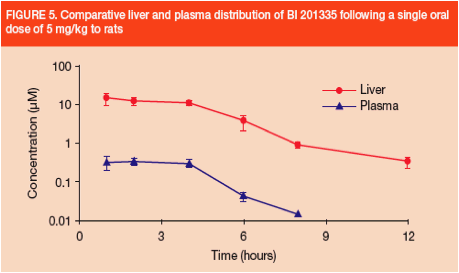
· In rats, following a single oral dose, BI 201335 was found at much higher concentrations in liver than in plasma. Liver and plasma levels decreased roughly in parallel (Figure 5)
- The rat liver:plasma AUC ratio was 42
· Based on consistent exposure in multiple species, BI 201335 was predicted to have good PK in humans, as well as a favorable liver distribution
- These preclinical data are consistent with the long apparent half-life observed in human trials, allowing BI 201335 to be dosed once daily
REFERENCES
1. Kukolj G, et al. BI 201335, a potent HCV NS3 protease inhibitor, in treatment-naïve and -experienced chronic HCV genotype-1 infection: genotypic and phenotypic analysis of the NS3 protease domain. The 44th Annual Meeting of the European Association for the Study of the Liver (EASL); Copenhagen, Denmark; 2009. Abstract 954.
2. Kukolj G, et al. Characterization of resistant mutants selected in vitro by the HCV NS3/4A protease inhibitor BI 201335. The 45th Annual Meeting of the European Association for the Study of the Liver (EASL); Vienna, Austria; 2010. Abstract 758.
3. Manns MP, et al. Safety and antiviral activity of BI 201335, a new HCV NS3 protease inhibitor, in treatment-naïve patients with chronic hepatitis C genotype 1 infection given as monotherapy and in combination with peginterferon alfa-2a (P) and ribavirin (R). The 59th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); San Francisco, CA, USA; 2008. Abstract 1849.
4. Manns MP, et al. Safety and antiviral activity of BI 201335, a new HCV NS3 protease inhibitor, in combination therapy with peginterferon alfa-2a (P) and ribavirin (R) for 28 days in P+R treatment-experienced patients with chronic hepatitis C genotype 1 infection. The 59th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); San Francisco, CA, USA; 2008. Abstract 1882.
5. Sulkowski M, et al. SILENC-1: early antiviral activity and safety of BI 201335 combined with peginterferon alfa-2a and ribavirin in treatment-naïve patients with chronic genotype 1 HCV infection. The 60th meeting of the American Association for the Study of Liver Diseases (AASLD); Boston, MA, USA; 2009. Abstract LB3.
6. Sulkowski M, et al. SILEN-C2: early antiviral activity and safety of BI 201335 combined with peginterferon alfa 2a and ribavirin (PegIFN/RBV) in chronic HCV genotype-I patients with non-response to PegIFN/RBV. The 45th Annual Meeting of the European Association for the Study of the Liver (EASL); Vienna, Austria; 2010. Late-breaker abstract.
|
| |
|
 |
 |
|
|