 |
 |
 |
| |
Virologic Response Rates Following 4 Weeks of Filibuvir in Combination with Pegylated Interferon Alfa-2a and Ribavirin in Chronically-Infected HCV Genotype-1 Patients
|
| |
| |
Reported by Jules Levin
EASL Apr 14-18 2010 Vienna Austria
I. Jacobson,1 P.J. Pockros,2 J. Lalezari,3 E. Lawitz,4 M. Rodriguez-Torres,5 E. DeJesus,6 F. Haas,7 C. Martorell,8 R. Pruitt,9 V.S. Purohit,10 S. Srinivasan,10 S. Jagannatha,10 K. Rana,10 J. Hammond10
1Weill Cornell Medical College, New York, NY, USA; 2The Scripps Clinic, La Jolla, CA, USA; 3Quest Clinical Research, San Francisco, CA, USA; 4Alamo Medical Research, San Antonio, TX, USA; 5Fundacion de Investigacion de Diego and Ponce School of Medicine, Santurce, PR, USA; 6Orlando Immunology Center, Orlando, FL, USA;
7University of Oklahoma-Schusterman Clinic, Tulsa, OK, USA; 8The Research Institute, Springfield, MA, USA; 9Nashville Medical Research Institute and Maria Nathanson Center of Excellence at Saint Thomas Hospital, Nashville, TN, USA; 10Worldwide Biopharmaceuticals, Specialty Care, Pfizer, New London, CT, USA
AUTHOR CONCLUSIONS
· All doses of filibuvir were well tolerated, with the incidence and severity of AEs and laboratory abnormalities similar to pegIFN/RBV and placebo.
· In treatment-naïve HCV genotype 1-infected patients, the addition of filibuvir (200, 300 or 500 mg BID) to a regimen of pegIFN/RBV for 4 weeks resulted in a significantly greater proportion of patients achieving RVR compared with pegIFN/RBV and placebo.
· The effect of filibuvir on HCV RNA concentrations persisted following cessation of triple therapy on Day 28. Following the achievement of undetectable HCV RNA during 4 weeks of triple therapy, the majority of filibuvir-treated patients remained undetectable through Week 48.
· As a result of the high relapse rates in the filibuvir arms, the response rates at Week 60 (SVR-12) were similar for the filibuvir and placebo groups. High relapse rates were reported from another study of a similar design (4 weeks triple therapy followed by 44 weeks pegIFN/RBV) and likely reflect the short duration of triple
combination therapy.6
· The impact of longer durations (24 weeks) of triple therapy on relapse SVR rates is being evaluated in an ongoing Phase 2b study in treatment-naïve patients.
ABSTRACT
Background: Filibuvir (FLV) is a non-nucleoside inhibitor of HCV polymerase that has shown >2 log10 IU/mL reductions in HCV RNA as monotherapy. In combination with pegIFN and RBV, 60-75% of FLV-treated patients
achieved a rapid virologic response (RVR) following 4 weeks of triple therapy. The outcome of these patients through Week 60 is reported here.
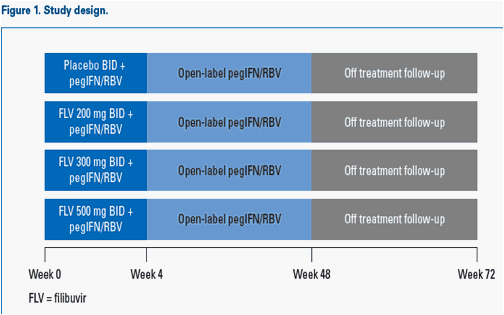
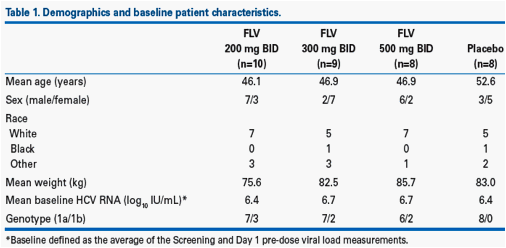
Methods: Treatment-naïve, HCV genotype-1 patients were randomised (1:1:1:1) to receive FLV (200, 300, or 500 mg BID) or placebo (PBO) in combination with pegIFN alfa-2a (180 µg/week) and RBV (1,000-1,200 mg daily) for 4 weeks. Patients continued on open-label pegIFN/RBV from Weeks 5-48.
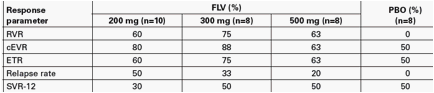
Results: Thirty-five patients were randomised, 34 completed 4 weeks triple therapy and 21 completed 48 weeks of pegIFN/RBV and 12 weeks of follow-up. Two patients (both PBO) discontinued due to AEs. Virologic response rates from the FLV and PBO groups are shown in the table below (all patients who completed Week 4 included). Up to 75% of FLV-treated patients achieved a RVR, and the majority remained undetectable on pegIFN/RBV therapy with 63-88% and 60-70% of all FLV-treated patients undetectable at Weeks 12 and 48, respectively. However, 20-50% of those FLV-treated patients undetectable at Week 48 relapsed by Week 60 compared to 0% of PBO-treated patients. As a result, response rates at Week 60 (SVR-12) were similar for the FLV and PBO treated groups.
Conclusions: FLV in combination with pegIFN and RBV for 4 weeks was well tolerated and resulted in higher on-treatment virologic response rates relative to pegIFN/RBV alone. However, longer durations of triple therapy will be required to assess the potential for reduced rates of relapse and improvement in SVR rates. A study evaluating 24 weeks of FLV/pegIFN/RBV is currently underway.
BACKGROUND
More effective and better tolerated therapies are needed for chronic hepatitis C virus (HCV) infection. Among the direct acting anti-HCV agents in development is the non-structural 5B (NS5B) non-nucleoside polymerase inhibitor filibuvir (formerly PF-00868554).
Filibuvir has potent in-vitro antiviral activity, with an overall mean EC
50 against HCV genotype 1 replicons of 0.059 µM.1 In-vitro data indicate equipotent activity against genotypes 1a and 1b.1
The safety, tolerability, pharmacokinetics and antiviral activity of filibuvir monotherapy have been investigated in HCV-infected patients in dose ranging studies.2,3
- Filibuvir potently inhibited viral replication in a dose-dependent manner with mean maximum HCV RNA
reductions ranging from -0.97 log10 IU/mL with 100 mg filibuvir BID to -2.30 log10 IU/mL with 700 mg filibuvir BID in treatment-naïve patients.2,3
- A -2.20 log10 IU/mL reduction was achieved with 450 mg filibuvir BID in treatment-experienced patients.3
In combination with pegylated interferon alfa-2a (pegIFN) and ribavirin (RBV), 60-75% of filibuvir-treated patients achieved a rapid virologic response (RVR) following 4 weeks of triple therapy.4
OBJECTIVES
To assess the antiviral activity, safety and tolerability of filibuvir (200-500 mg BID) when administered in combination with pegIFN/RBV over 28 days in treatment-naïve HCV genotype 1-infected patients.
Patients continued to receive pegIFN and RBV for an additional 44 weeks, and the outcome of patients through to Week 60 is reported here.
METHODS
Study design
This was a randomised (1:1:1:1), double-blind, placebo-controlled, dose-ranging, parallel-group, multicentre study (Figure 1).
Patients received filibuvir 200, 300 or 500 mg BID or placebo combined with:
- Pegylated interferon alfa-2a (Pegasys®) 180 µg/week
- Ribavirin (Copegus®) 1,000-1,200 mg/day.
The protocol was reviewed and approved by an independent ethics committee and all patients gave written informed consent.
Results through to Week 60 are described here.
Key inclusion criteria
· Males and females aged 18-65 years.
· A diagnosis of chronic HCV genotype 1 infection for at least 6 months.
· HCV RNA ≥100,000 IU/mL at screening.
· HCV treatment-naïve.
Key exclusion criteria
· HBV or HIV co-infection.
· Evidence of severe or decompensated liver disease.
· Liver disease unrelated to HCV-infection.
HCV RNA measurements
· Samples for HCV RNA determination were collected at Screening, Day 1 (0, 1, 2 and 6 hours post-dose), Day 4, Weeks 1, 2, 3 and 4, monthly thereafter through Week 48, and at Weeks 60 and 72.
· HCV RNA levels were quantified using the Roche COBAS Taqman assay (LLD = 25 IU/mL).
· Virologic stopping criteria:
- <2.0 log reduction in HCV RNA from baseline at Week 12
- Detectable HCV RNA at Week 24.
NS5B sequence analysis
· Samples from all filibuvir-treated patients who did not achieve a RVR by Week 4 underwent population
analysis of the HCV NS5B sequence.
· The entire NS5B region was sequenced using standard methodologies.
· Sequence analysis was performed by Lab21 Inc. (Cambridge, UK).
Safety and tolerability assessments
· Physical examinations, electrocardiograms, vital sign measurements and clinical laboratory tests were
performed and adverse events (AEs) were monitored weekly until Week 4, and monthly thereafter.
Data analyses
· Safety analyses were performed on the intent-to-treat population.
· Efficacy analyses were conducted on the per protocol population, including all patients who completed through to Week 4.
RESULTS
Patient demographics
· Demographic parameters and baseline patient characteristics in each treatment group are listed in Table 1.
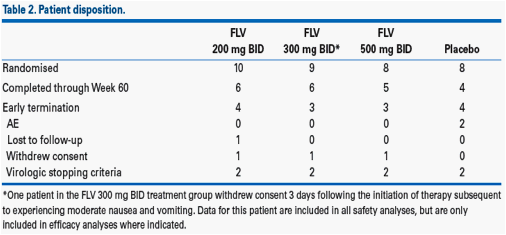
· Patient disposition is shown in Table 2.
Efficacy
· All doses of filibuvir significantly increased the proportion of patients achieving undetectable HCV RNA at Week 4 (RVR) when added to a regimen of pegIFN/RBV compared with placebo and pegIFN/RBV (all p<0.05; Figure 2).
- Following 4 weeks of triple therapy, 63-88% of filibuvir-treated patients achieved a complete EVR (cEVR), compared with 50% of patients receiving placebo and pegIFN/RBV during Weeks 1-4.
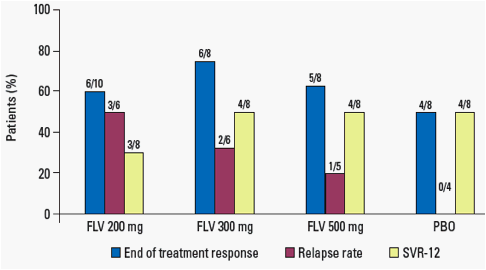
· However, 20-50% of those filibuvir-treated patients who were undetectable at Week 48 relapsed by Week 60, compared with 0% in the placebo and pegIFN/RBV group.
- As a result, response rates at Week 60 (SVR-12) were similar for the filibuvir and placebo groups (Figure 3).
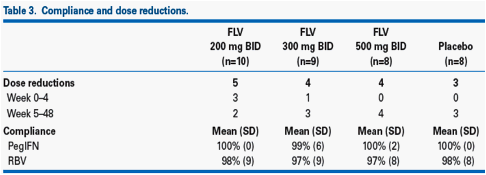
· The higher relapse rates observed in the filibuvir arms were unrelated to dose reductions or treatment compliance (Table 3).
NS5B sequence
· 9/26 filibuvir-treated patients failed to achieve an RVR at Week 4 and were included in the NS5B populationbased sequence analysis.
- 6/9 patients had mutations at position 423 at Weeks 4, 8 or 12. Of these:
· 5/6 had a null response (<2 log drop at Week 12)
· The one remaining patient had a -2.15 log drop at Week 12.
- Filibuvir resistance-associated mutations could not be detected in 3/9 patients at Weeks 4, 8 or 12 (primarily due to inability to amplify vRNA).
· All three patients achieved undetectable HCV RNA by Week 12.
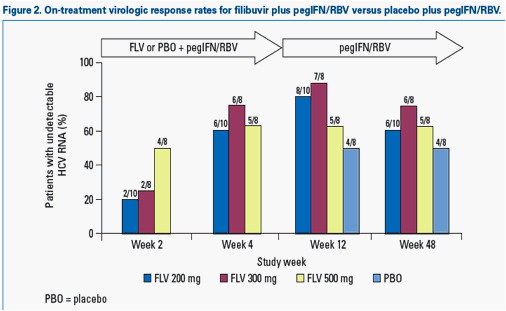
Figure 3. End-of-treatment response, relapse rate and SVR-12.
· no M423 variants were observed at baseline.
· NS5B sequence analysis for FLV-treated patients who relapsed is ongoing. Those subjects with FLV-resistant variants at Week 60 will be followed to assess reversion to wild-type.5
Safety and tolerability
· The addition of filibuvir to pegIFN/RBV was well tolerated over a period of 28 days. Following the discontinuation of filibuvir on Day 28, the filibuvir treatment groups remained comparable with the placebo group with respect to AEs, laboratory abnormalities and pegIFN or RBV dose reductions.
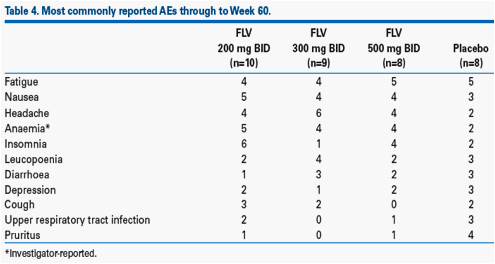
· The most frequently reported AEs were fatigue, nausea and headache (Table 4). There were no notable trends in AEs across treatment groups.
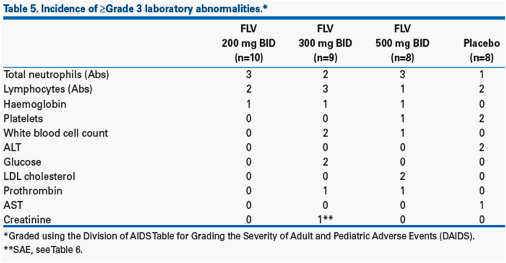
· There were no trends in the incidence of ≥Grade 3 laboratory abnormalities (Table 5).
· Two patients discontinued from the study due to an AE; both discontinuations occurred in the placebo group.
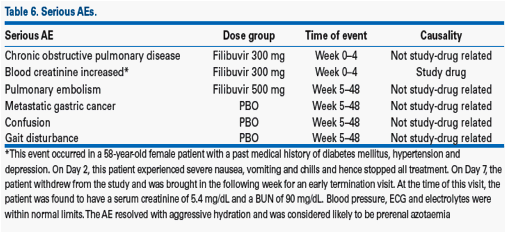
· There were six serious AEs reported in five patients (Table 6).
REFERENCES
1. Shi ST, et al. Preclinical characterization of PF-00868554, a potent nonnucleoside inhibitor of the hepatitis C virus RNA-dependent RNA
polymerase. Antimicrob Agents Chemother 2009;53:2544-2552.
2. Hammond JL, et al. Antiviral activity of the HCV polymerase inhibitor PF-00868554 administered as monotherapy in HCV genotype 1
infected patients. Poster LB11 presented at AASLD 2008.
3. Pfizer, Inc. data on file.
4. Jacobson I, et al. Antiviral activity of filibuvir in combination with pegylated interferon alfa-2a and ribavirin for 28 days in treatment naïve patients chronically infected with HCV genotype 1. Poster 1052 presented at EASL 2009.
5. Mori J, et al. Genotypic characterisation of filibuvir (PF-00868554) resistance in patients receiving four weeks co-administration of filibuvir with pegIFN/RBV (12 week analysis). Oral 993 presented at EASL 2010.
6. Pockros P, et al. High relapse rate seen at week 72 for patients treated with R1626 combination therapy. Hepatology 2008; 48;1349-1350
|
| |
|
 |
 |
|
|