 |
 |
 |
| |
Resistance Profile Of ABT-333 And Relationship To Viral Load Decrease In Patients Treated In Combination With Peg-interferon And Ribavirin For 28 Days.
|
| |
| |
Download the PDF Here
Reported by Jules Levin
EASL 2010 Vienna Austria Apr 14-18
Tim Middleton, Yupeng He, Jill Beyer, Rajeev Menon, Cheri E. Klein, Daniel Cohen and Christine Collins
Abbott Laboratories, Abbott Park, IL, USA
AUTHOR SUMMARY & CONCLUSIONS
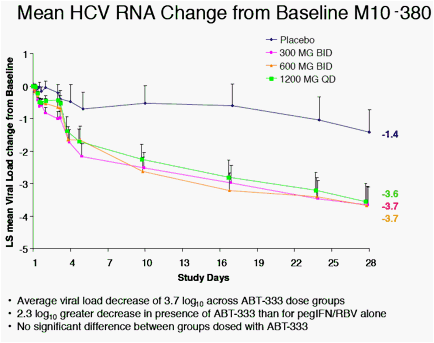
· ABT-333 in combination with pegIFN/RBV resulted in a 2.3 log10 greater decrease in viral titer than pegIFN/RBV alone.
· Resistant variants emerged on treatment with ABT-333, but the emergence of resistance did not appear to impact continued response to therapy, suggesting that resistant variants are still susceptible to the combination of ABT-333 + pegIFN/RBV.
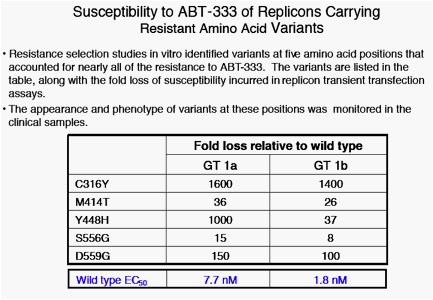
· In vitro resistance selection implicated variants at amino acids 316, 414, 448, 556 and 559. Variants at these positions were found in almost all of the isolates where phenotypic resistance was observed, though this analysis does not preclude the existence of other variants that may have contributed to the loss of susceptibility to ABT-333.



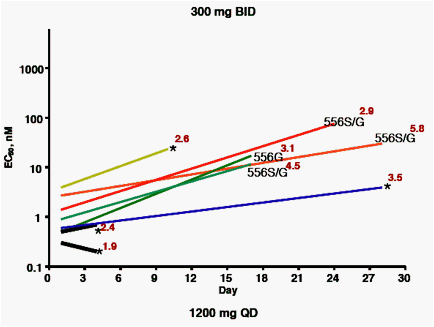
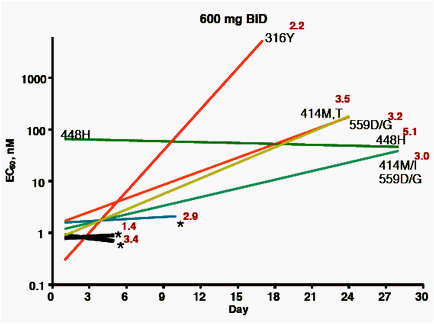
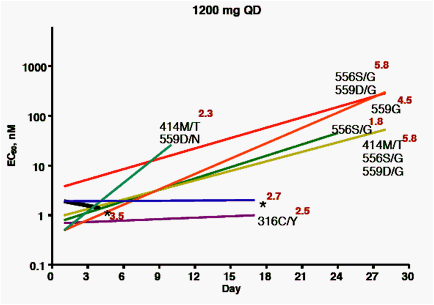
Samples taken before dosing, or at days 4, 10, 17, 24 or 28 days after the initial dose were analyzed for viral titer, ABT-333 EC50 against NS5B
isolates cloned into replicons, and for the presence of variants previously demonstrated to confer resistance to ABT-333.
A, B, C: plots by dose group, showing:
· Baseline and maximum EC50 observed during the treatment period (beginning and end of lines)
· Known resistance variants observed at time of maximum EC50 (* = no variant noted)
· Viral load at time of maximum EC50 (log10, in red)
Subjects with short time courses plotted (black lines) had viral titers that were too low to permit amplification of the NS5B gene at later time points
SUMMARY
· 28 of 30 baseline samples had EC50 between 0.3 - 3.9 nM. One
baseline EC50 = 10 nM, with no known resistance variants detected. The remaining baseline isolate EC50 = 66 nM; this isolate encoded a histidine at amino acid 448, a known resistant variant.
· Variants noted at later time points at amino acids 414, 448, 556 and 559 were associated with EC50 values from 10 - 400 nM.
· An isolate with complete conversion from Cys to Tyr at amino acid
316 increased EC50 to >3000 nM. Known resistance variants were associated with an increase over time in EC50
· Resistance variants were distributed throughout the range of viral load decreases observed, indicating that the presence of resistance variants did not impact the response to therapy.
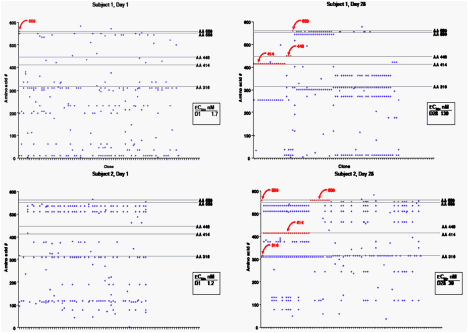
Clonal Sequence Analysis
Clonal isolates of the polymerase gene were sequenced to:
· Assess the extent of evolution of quasispecies with ABT-333 treatment.
· Determine whether signature resistance variants identified by population sequencing were linked in clones.
· Establish the predictability of in vitro resistance selection for in vivo studies with ABT-333
Summary
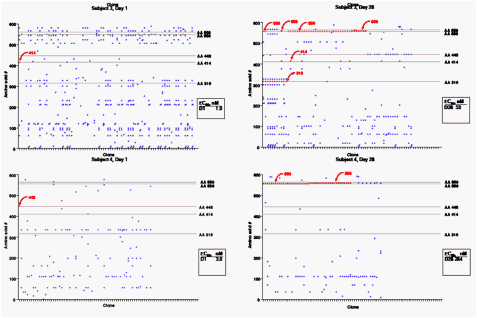
· An average of 72 clones were sequenced per sample tested. The plots show examples from four subjects, with a comparison of each clone to the consensus sequence for all of the clones from that isolate for days 1 and 28.
· 31 of 54 clinical isolates (57%) contained at least one signature resistance variant.
· Variants were relatively uniformly distributed between the five amino acid positions.
· Resistance variants accumulated with time of treatment. Six percent of clones from baseline isolates contained resistance variants, with 19% of clones at day 10 and and 36% at days 24 or 28 containing resistance variants.
· The average number of variants associated with a sample also increased over time of treatment (1.6, 25 and 39% at days 1, 10 and 24/28). There was a general increase in EC50 as the proportion of resistance variants increased, though number or position of variant did not predict potency loss. Note, for instance that subject 3 on day 28 contained resistance variants in 46 of 66 clones, including 11 clones encoding C316Y, yet EC50 only increased from 1 nM at baseline to 53 nM at day 28.
· Clonal sequencing showed a shift in the quasispecies found in the population on treatment with ABT-333 for most of the subjects.
· Linkage of resistance variants occurred in a few clones, but was relatively rare. In most instances where multiple resistance variants were noted in an isolate by mixed population sequencing, clonal sequencing indicated that the variants were found on different clones.
|
| |
|
 |
 |
|
|