| |
Sexually Transmitted Diseases Among Users of Erectile Dysfunction Drugs: Analysis of Claims Data
|
| |
| |
" The OR for HIV infection was 3.32 (CI, 2.38 to 4.36) in the year before and 3.19 (CI, 2.11 to 4.83) in the year after an ED drug prescription was filled. Significant changes in STD rates from the year before to the year after the first ED drug prescription was filled were not documented ......use of ED drugs by middle-aged and older patients may serve as a simple screening tool for physicians to use in identifying those patients who may benefit from reminders about safe sexual practice......Although we found no evidence that relative rates of STDs among users of ED drugs increased after initiating ED drug therapy, limitations of our data do not allow us to dismiss the possibility that increased availability of ED treatments (perhaps through more generous insurance coverage or generic entry in the future) may directly lead to increases in STDs.(see discussion).......In addition to the limitations raised in the previous section, our analysis has several others. First, we did not conduct a randomized, controlled trial, which would have been ideal for assessing the direct link between ED drug use and STD risk. Second, we identified users of pharmacologic ED treatments and rates of STDs from insurance claims data."
Men on Viagra, Cialis Show Triple Rate of Sex Diseases (HIV) in Study - full text below
July 06, 2010, 12:04 AM EDT
By Nicole Ostrow
July 6 (Bloomberg) -- Men taking drugs for sexual potency showed almost triple the rate of sexually transmitted diseases compared with those not taking the medications, a Harvard University study found.
The results, from an analysis of the health insurance claims of men aged 40 and older, may have more to do with the nature of the men using the impotence drugs than with the medicines leading them to have riskier sex, the research report said. The study, looking at men taking Pfizer's Inc. Viagra and Eli Lilly & Co.'s Cialis, was published today in the Annals of Internal Medicine.
The higher rate of infections was seen in the year before and after the men started taking the prescription medicines, according to the analysis. That suggests that users of drugs to treat erectile dysfunction, which also include Bayer AG's Levitra, may be more likely to engage in unsafe sex than nonusers, lead study author Anupam Jena said.
"Younger people have more sex partners than older folks," said Jena, a medical resident in internal medicine at Massachusetts General Hospital and Harvard Medical School in Boston, in a June 29 telephone interview. "But per sexual encounter, the actual safeness of the sex is probably lower among older folks in the sense that they don't use condoms," he said.
About 19 million new sexually spread infections occur each year in the U.S., almost half of them among people ages 15 to 24 years old, according to the U.S. Centers for Disease Control and Prevention.
HIV in Middle-Aged
Still, people aged 40 to 49 accounted for the largest proportion of newly diagnosed HIV/AIDS cases, 27 percent, in 2007, according to the CDC. Those 50 to 59 accounted for 13 percent, while those over the age of 60 accounted for 4 percent.
"Old folks can contract STDs and we need to be vigilant about it," Jena said.
The researchers looked at health insurance claims from 44 large U.S. employers from 1997, one year before Viagra was introduced, through 2006. They examined the claims of men over the age of 40 to see if they were prescribed erectile dysfunction drugs, finding 33,968 who used the impotence pills and about 1.38 million who did not.
The research found that in 2006, 3.6 percent of the men used Viagra from New York-based Pfizer; 1.7 percent took Cialis from Indianapolis-based Lilly, and 1 percent were getting Levitra from Leverkusen, Germany-based Bayer.
Sexual Behavior Unknown
The authors weren't able to determine how many of the men were married and how many were heterosexual or other information on sexual behavior.
In the year before taking the pills, users of ED drugs had an overall STD rate of 214 per 100,000 people, the study found. That gave them a 2.8 times greater risk of developing a sexually spread infection than men who didn't take the drugs.
That decreased slightly in the year after, when pill-takers had a 2.65 times higher risk than non-takers, the study showed.
The risk of getting HIV in the year before taking the pills was 3.32 times higher in drug-takers and 3.19 times greater in the year after, compared with those not taking the pills, they said. Users of the medicines also had higher rates of chlamydia.
Seeking Treatment
The rates of HIV may be higher than other infections because the men seek treatment from their doctor for HIV symptoms like fever and weight loss, Jena said. Men may go to free clinics to treat other sexually transmitted diseases like herpes and gonorrhea, he said.
An editorial accompanying the research article, entitled "Life Begins at Forty," urged doctors to advise older patients to remember safe-sex precautions if they ask for erectile dysfunction drugs.
"STD counseling should not stop at age 40," said the editorial written by Thomas Fekete, professor of medicine at Temple University in Philadelphia.
Sexually Transmitted Diseases Among Users of Erectile Dysfunction Drugs: Analysis of Claims Data
Annals of Internal Medicine July 6 2010
Anupam B. Jena, MD, PhD; Dana P. Goldman, PhD; Amee Kamdar, PhD; Darius N. Lakdawalla, PhD; and Yang Lu, PhD
Abstract
Background: Pharmacologic treatments for erectile dysfunction (ED) have gained popularity among middle-aged and older men. Increased sexual activity among those who use these drugs raises concerns about sexually transmitted diseases (STDs).
Objective: To examine the rates of STDs in men who use and do not use ED drugs.
Design: Retrospective cohort study.
Setting: Database of claims from 1997 to 2006 for 1 410 806 men older than age 40 years with private, employer-based insurance from 44 large companies.
Patients: 33 968 men with at least 1 filled prescription for an ED drug and 1 376 838 patients with no prescription.
Measurements: STD prevalence among users and nonusers of ED drugs.
Results: Users of ED drugs had higher rates of STDs than nonusers the year before initiating ED drug therapy (214 vs. 106 annually per 100 000 persons; P = 0.003) and the year after (105 vs. 65; P = 0.004). After adjustment for age and other comorbid conditions, users of ED drugs had an odds ratio (OR) for an STD of 2.80 (95% CI, 2.10 to 3.75) in the year before initiating drug therapy; the OR was 2.65 (CI, 1.84 to 3.81) in the year after. These differences were largely due to infections with HIV. The OR for HIV infection was 3.32 (CI, 2.38 to 4.36) in the year before and 3.19 (CI, 2.11 to 4.83) in the year after an ED drug prescription was filled. Significant changes in STD rates from the year before to the year after the first ED drug prescription was filled were not documented (adjusted OR for STD for users before vs. after the first ED drug prescription was filled, 0.96 [CI, 0.87 to 1.06]).
Limitation: Selection bias precludes conclusions about whether use of ED treatments directly leads to increases in STDs.
Conclusion: Men who use ED drugs have higher rates of STDs, particularly HIV infection, both in the year before and after use of these drugs. The observed association between ED drug use and STDs may have more to do with the types of patients using ED drugs rather than a direct effect of ED drug availability on STD rates. Counseling about safe sexual practices and screening for STDs should accompany the prescription of ED drugs.
Primary Funding Source: RAND Roybal Center, National Institutes of Health, and Agency for Healthcare Research and Quality.
Editors' Notes
Context
* Nearly 40% of men aged 57 to 85 years have erectile dysfunction (ED), and pharmacologic treatments are popular. Risks for sexually transmitted diseases (STDs) may accompany the increased sexual activity that these drugs enable.
Contribution
* The comparison of STD prevalence in men who did and did not have a new prescription for an ED drug from 1997 to 2006 showed that both in the year before and the year after drug initiation, men with a prescription had higher rates of HIV infection and chlamydia than those with no prescription.
Implication
* Risk assessment for STDs and counseling about safe sexual practices should accompany prescription of ED drugs.
-The Editors
Pharmacologic treatments for erectile dysfunction (ED) have gained widespread popularity among middle-aged and older men in recent years. Driven largely by the high prevalence of erectile difficulties in this population (1-8), rates of sildenafil use reportedly reached 1.4% in the commercially insured population by 2002 (9). This is perhaps not surprising, because nearly 40% of men aged 57 to 85 years have some degree of ED (8). Although their clinical efficacy has been well documented, little is known about the relationship between ED treatments and the prevalence of sexually transmitted diseases (STDs). In light of growing evidence for the increasing incidence of STDs, including AIDS, diagnosed at an older age (10-14), ED drugs have received attention for their possible contribution to these trends (13, 14). A recent study found that widowhood in older men, but not older women, was associated with higher rates of STDs, especially after the introduction of sildenafil in 1998 (15).
Although middle-aged and older adults generally take fewer risks with their health, their decreased need for contraception may imply less-than-optimal safe sexual practices compared with younger populations (16). For example, previous research suggests that condom use declines with age (16, 17), and among at-risk populations, persons older than 50 years are one sixth as likely to use condoms during sex and one fifth as likely to have been tested for HIV compared with persons in their 20s (18). Moreover, a survey of primary care physicians revealed that most physicians rarely or never discuss sexual risk factor reduction with their middle-aged and older patients (19). These facts are particularly important in light of the emergence of ED drugs, which have improved sexual function among older men.
Several small studies of men who have sex with men have investigated the connection between pharmacologic ED treatments and STDs (20-24). In this community, ED drug use is associated with high-risk sexual behavior, such as unprotected anal sex (20-22). Users of ED drugs also report more recent sex partners and higher rates of STDs than nonusers (21-23). Although the measured outcomes of these studies probably reflect selection bias among users rather than the effect of ED drugs per se, these studies nonetheless highlight a group within the community of men who have sex with men who are at high risk for STDs.
In light of these findings and the growing use of pharmacologic treatments for ED, we investigated the relationship between STDs and ED drug use in a comprehensive, large sample of privately insured, middle-aged and older adult male beneficiaries. Large data sets are required to examine STD patterns because in the general population, STDs are still rare, and even more so among middle-aged and older adults (15). For men older than 40 years, we retrospectively calculated STD rates among users of ED drugs over 24 months: 12 months before and 12 months after the first ED drug prescription was filled. We compared these rates with a reference group of nonusers during the same 12-month period before and after users of ED drugs filled their first prescription. We hypothesized that users of ED drugs would have higher rates of STDs than nonusers both before and after the first ED drug prescription was filled. A confirmation of this hypothesis would suggest that men who request ED drugs would be at higher risk for contracting or already having an STD and may therefore benefit from closer monitoring of risky sexual behavior and renewed discussions about safe sexual practices. By using the first 12 months in our sample as a baseline, we also examined whether users of ED drugs had larger increases in STD rates after filling the first prescription compared with nonusers-a "difference-in-difference" approach. Evidence of such a pattern would be consistent with, although not conclusive of, ED drugs directly leading to higher rates of STDs, perhaps by facilitating sexual activity among those who were previously less sexually active.
Discussion
Since the introduction of sildenafil in 1998, pharmacologic treatments for ED have gained increasing popularity among middle-aged and older men. We investigated the relationship between ED drug use and STDs in a comprehensive, large sample of privately insured, middle-aged and older male beneficiaries. Generally, we found that users of ED drugs had higher rates of STDs compared with nonusers, both before and after initiating ED drug therapy. In addition, we found that the relative difference in STD rates between users and nonusers was unchanged in the year after the first ED drug prescription was filled, suggesting that the observed association between ED drug use and STDs may have more to do with the types of patients using ED drugs rather than a direct effect of ED drug availability on STD rates.
At a minimum, use of ED drugs seems to correlate with higher-risk sexual behavior, either in the number or type of sexual encounters (neither of which we could observe in our data). The simple fact that STD rates are higher among users of ED drugs at the very least suggests a particular subset of men who are at higher risk for STDs and who may benefit from renewed physician conversations about safe sexual practices. This is particularly relevant because most primary care physicians rarely discuss sexual risk factor reduction with middle-aged and older patients (19), and only 9% of adults aged 40 to 80 years report that a physician asked them about their sexual health during a routine physician visit in the past 3 years (28). Put differently, use of ED drugs by middle-aged and older patients may serve as a simple screening tool for physicians to use in identifying those patients who may benefit from reminders about safe sexual practice. This finding coincides with other researchers' recommendations that physicians should include discussions about sexual health in conversations with older patients (29). Of importance, although conversations about safe sexual practices may be warranted, routine STD testing of men requesting ED drugs may not be. Although the relative difference between users and nonusers of ED drugs is substantial, STD prevalence in older adults remains low, and broad STD testing of those requesting ED drugs from their physician would probably not be cost-effective. For example, with annual average rates of STD prevalence among users of 160 per 100 000 men, nearly 625 men who request ED drugs would need to be screened to identify a single STD case.
Although we found no evidence that relative rates of STDs among users of ED drugs increased after initiating ED drug therapy, limitations of our data do not allow us to dismiss the possibility that increased availability of ED treatments (perhaps through more generous insurance coverage or generic entry in the future) may directly lead to increases in STDs. To address this possibility, future research might use the introduction of sildenafil in 1998 to analyze whether persons with medical conditions predisposing to ED witnessed higher growth in STDs between the months before and after the introduction of sildenafil. This was not possible with our data because most persons entered our sample after the introduction of sildenafil so STD rates before sildenafil could not be calculated. If a direct effect between ED drug use and STD risk exists, a natural question is what steps, if any, should be taken to ensure responsible use of these treatments. For example, health plans may consider increasing copays for these treatments or manufacturers may consider including information about safe sexual practices as part of package inserts or direct-to-consumer advertising. Physicians may also remind their older adult patients about responsible use of these drugs.
In addition to the limitations raised in the previous section, our analysis has several others. First, we did not conduct a randomized, controlled trial, which would have been ideal for assessing the direct link between ED drug use and STD risk. Second, we identified users of pharmacologic ED treatments and rates of STDs from insurance claims data. Measured ED drug utilization may not capture drugs purchased outside of a patient's health plan, and measured STD rates may miss visits to anonymous clinics. For example, one study showed that sildenafil is readily available over the Internet without the need for a physician visit (30). Third, although our claims data include information on many covered lives, the prevalence of STDs is still low and precludes a highly powered analysis at the individual STD level; a more refined analysis would more precisely target those diseases that screening efforts would be best targeted toward. More generally, further work may better characterize those users of ED drugs who are at highest risk for an STD. Screening, whether in the form of brief conversations or formal STD testing, would be most effective if targeted toward those at highest risk.
Results
Sildenafil was approved for use in ED by the U.S. Food and Drug Administration in March 1998. From 1998 to 2003, sildenafil use among men older than 40 increased from 4.3% to 6.3% in our sample. The U.S. Food and Drug Administration approved vardenafil and tadalafil in September 2003 and December 2003, respectively. With the arrival of these competing drugs, sildenafil use decreased to 3.7% in 2006, as vardenafil and tadalafil steadily gained market share. In 2006, the last year of our data set, sildenafil remained the market leader of ED drugs; 3.6% of men older than 40 years used sildenafil, 1.0% used vardenafil, and 1.7% used tadalafil.
Table 1 presents descriptive statistics of users and nonusers of ED drugs in our study. It shows that, in general and not surprisingly, users of ED drugs were older and had higher rates of chronic disease. Overall rates of STDs in the year before the reference start date were substantially higher among those who ended up using an ED drug. Note that HIV is substantially more prevalent than other diseases; this is because HIV is not curable, and therefore the prevalence is high. Although differences in individual STDs (except for chlamydia and HIV) are not statistically significant because of low power, after we pooled together all STDs and excluded HIV, users of ED drugs still had statistically significant higher rates of STDs before initiating use of drugs than nonusers previously in the same period.
Figure 1 extends our comparison of users and nonusers of ED drugs by presenting observed and predicted rates of STDs for both groups. Predicted rates are derived from our estimated logistic model. Sexually transmitted diseases are presented at the quarterly level, with the first 4 quarters reflecting the year before reference users filled their first prescription (quarters -4 to -1) and the last 4 quarters reflecting the year after (quarters 1 to 4). The top 2 panels in Figure 1 include each STD, whereas the bottom 2 panels exclude HIV to more clearly display differences in the remaining STDs. Rates are per 100 000 beneficiaries.
Both Table 1 and Figure 1 demonstrate that users of ED drugs had higher rates of HIV, chlamydia, gonorrhea, and syphilis in the 12 months before filling their first ED drug prescription, although only HIV and chlamydia were statistically significant in this period (P < 0.001 for both). One year after filling the first prescription for an ED drug (fourth quarter), users continued to have higher cumulative rates of HIV, chlamydia, syphilis, and herpes, although only HIV and chlamydia were significant in this period (P < 0.001 for both). After we grouped together all STDs and excluded HIV, users of ED drugs continued to have statistically significant higher cumulative rates of STDs compared with nonusers both just before filling the first ED drug prescription (that is, by quarter -1 in Figure 1; P = 0.03) and within 1 full year after (that is, by the eighth and final quarter) (P = 0.02).
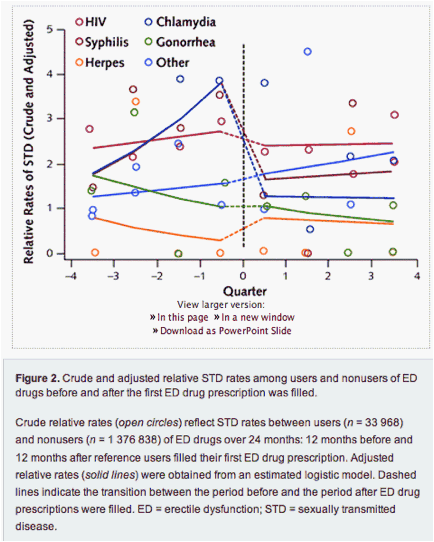
Figure 2 incorporates the information in Figure 1 and plots both crude and adjusted relative rates of STDs between users and nonusers of ED drugs. The relative rates are again presented quarterly. It shows that 1 year before filling the first ED drug prescription, users had higher adjusted relative rates of HIV, chlamydia, and gonorrhea, of which only HIV and chlamydia were significant in this period (P < 0.001 for both). In the quarter before filling the first ED drug prescription (quarter -1), users had adjusted relative rates of chlamydia of almost 3 (P < 0.001), adjusted relative rates of HIV of approximately 2.5 (P < 0.001), and adjusted relative rates of gonorrhea of approximately 1.1 (P = 0.36). One year after filling the first prescription (eighth and final quarter), adjusted relative rates of HIV were nearly unchanged. Chlamydia exhibited a different pattern: Compared with nonusers, users of ED drugs had crude and adjusted relative rates that increased in the quarter immediately after the first ED drug prescription was filled but declined by the end of 12 months. In other words, although relative rates of chlamydia were higher among users both before and after they filled their first ED drug prescription, the relative rate was lower in the year after the first drug was filled than in the year before.
Figure 1 demonstrates that users of ED drugs had higher rates of certain STDs than nonusers both before and after initiating ED drug treatments. Figure 2 also suggests that compared with nonusers, users of ED drugs did not have a relative increase in STD rates in the year after filling the first ED drug prescription compared with the year before. Table 2 tests this formally and presents results from our difference-in-difference logistic model, which estimated differences in STD rates between users and nonusers and within both groups over time. The model accounted for the hierarchical longitudinal structure of our data by including random effects at the individual and employer levels and adjusted for covariates, such as age, disease, and calendar year of entry.
Table 2 shows that in the year before the first ED drug prescription was filled, users of ED drugs had an adjusted OR of an STD of 2.80 (P < 0.001) compared with nonusers. This was mainly driven by HIV (OR, 3.32; P < 0.001) and chlamydia (OR, 2.25; P < 0.09). In the year after the first ED drug prescription was filled, users continued to have a higher adjusted OR of an STD than nonusers (OR, 2.65; P < 0.001), this time driven only by HIV (OR, 3.19; P < 0.001). For both users and nonusers, there was essentially no change in STD rates from the year before to the year after the first ED drug prescription was filled. For example, the adjusted OR of an STD for users before compared with after was 0.96 (P = 0.40); for nonusers, it was 1.01 (P = 0.36). In other words, there was no within-group effect of ED drug use on STDs. Consistent with these between- and within-group effects, the difference-in-difference ORs for all STDs combined was statistically indistinguishable from an OR of 1 (OR, 0.95; P = 0.36). Although users of ED drugs had higher rates of STDs than did nonusers in both the year before and the year after the first reference ED drug prescription was filled, this difference between groups did not change across the 2 periods, calling into question whether the availability of ED drugs may have led to increased rates of STDs.
Sensitivity Analysis
We conducted a simple misspecification analysis to examine how misclassification of ED drug exposure would affect our results. For example, users of ED drugs may not have claims for these medications and could be incorrectly misclassified as nonusers. Similarly, nonusers of ED drugs could be incorrectly classified as users. Varying each of these probabilities (in particular, assuming that 10% of actual users of ED drugs were misclassified as nonusers and 20% of nonusers were incorrectly classified as users), we found that the between-group effect demonstrating differences in STD rates between users and nonusers was essentially unchanged, as was the absence of a within-group effect among users and nonusers. Results are available from the authors on request.
Methods
We assembled a data set of pharmacy and medical claims at the monthly level from 1997 to 2006 for 44 large U.S. employers. Because we were interested in STD rates among men most likely to use ED drugs, we restricted our sample to men older than 40 years (9). Our final data included 1 410 806 male beneficiaries continuously enrolled for 2 years (n = 67 718 688 person-months). The data were deidentified; therefore, the institutional review board at Massachusetts General Hospital exempted them from review.
The pharmacy claims incorporated all prescription drug claims, each with information on the type of drug, drug name, national drug code, dosage, and days supplied. The medical claims included the date of service, diagnosis, and procedure code. These data have been used elsewhere to examine the effect of benefit design on pharmacy spending (25), use of medication by chronically ill persons (26, 27), and specialty drugs (27). Although all types of health care encounters were captured-including inpatient, emergency, and outpatient services-our claims data excluded both the informal provision of prescription drugs by online or "black-market" suppliers, as well as prescriptions that were filled but not reported to the health plan. This is, of course, a possibility for drugs used to treat ED.
Our level of observation was an individual in 1 of 4 quarters of the year. In each quarter, we classified a person as using an ED drug if they filled 1 or more prescriptions for either sildenafil, tadalafil, or vardenafil in that quarter. We identified use of any of these drugs by searching the pharmacy claims data for both the generic and branded names of these drugs, as well as the national drug codes associated with them.
We flagged persons by quarter according to whether they had at least one claim for one of the following STDs: chlamydia, gonorrhea, herpes, HIV/AIDS, syphilis, or other (Haemophilus ducreyi infection, human papillomavirus infection, or lymphogranuloma venereum). Disease indicators for these STDs were identified in the medical claims according to International Classification of Diseases, Ninth Revision, diagnoses. (A full list of the codes that we used is available from the corresponding author).
We also constructed disease indicators for comorbid conditions, some of which might be associated with the use of ED drugs and the likelihood of an STD. Separate disease indicators identified the following conditions: anxiety, asthma, cancer, cardiac disease, congestive heart failure, chronic obstructive pulmonary disease, depression, diabetes, hypercholesterolemia, hypertension, stroke, and vascular disease. A beneficiary was determined to have 1 of these chronic conditions if their medical claims included 2 or more office visits with the corresponding International Classification of Diseases, Ninth Revision, code (available on request).
Statistical Analysis
We compared STD rates between users and nonusers of ED drugs, as well as STD rates before and after the first ED drug prescription was filled within users and nonusers (using the reference user to define the periods before and after the first ED drug was filled for nonusers). We estimated a logistic model of the following form:
Formula
in which: quarter = -4, -3, -2, -1, 1, 2, 3, 4; postperiod = indicator for quarter > 0 for reference users; user = indicator for ED drug user; and STD = indicator for presence of an STD.
The model included terms to define both differences between groups and changes within groups over time. This difference-in-difference approach estimates the difference in STD trajectories between users and nonusers of ED drugs before and after filling an ED drug prescription (presented as an odds ratio [OR] derived from coefficient b5). The difference-in-difference OR would be greater than 1 if, relative to nonusers, users of ED drugs had greater increases in STDs in the year after filling their first ED drug prescription compared with the year before. A difference-in-difference OR greater than 1 would be consistent with, although not conclusive of, a direct effect of ED drug availability on STDs. The logistic model was estimated separately for each STD and for all STDs combined, and we adjusted for covariates, such as age, disease, and calendar year of entry. We accounted for the hierarchical longitudinal structure of our data by including random effects at the individual and employer levels. Predicted probabilities from this adjusted model were also plotted alongside disease-specific prevalence data for users and nonusers. We used Stata, version 10 (StataCorp, College Station, Texas), for statistical analyses, and the 95% CI reflects 0.025 in each tail or P = 0.05.
Role of the Funding Source
The Bing Center for Health Economics and the RAND Roybal Center for Health Policy Simulation sponsored the research for this study. Dr. Jena received support from the National Institutes of Health through a Medical Scientist National Research Award Grant and from the Agency for Healthcare Research and Quality through a University of California, Los Angeles/RAND Training Grant. The funding sources had no role in the design, conduct, analysis, interpretation, and presentation of the data or in the decision to submit the manuscript for publication.
| |
| |
| |
|
|
|