 |
 |
 |
| |
Pooled Week 48 efficacy and safety results from ECHO and THRIVE, two double-blind, randomised, Phase III trials comparing TMC278 versus efavirenz in treatment-naïve, HIV-1-infected patients
|
| |
| |
Reported by Jules Levin
18th Intl AIDS Conference July 18-23 2010 Vienna Austria
C Cohen,1 JM Molina,2 P Cahn,3 B Clotet,4 J Fourie,5 B Grinsztejn,6 W Hao,7
M Johnson,8 M Saag,9 K Supparatpinyo,10 H Crauwels,11 L Rimsky,11
S Vanveggel,11 P Williams,11 K Boven12
1Community Research Initiative New England, Boston, USA; 2Dept Infect Dis, Saint-Louis Hospital and Univ Paris, France; 3Hospital Juan A Fernandez and Fundacion Huesped, Buenos Aires, Argentina; 4Hospital Universitari Germans Trias i Pujol and irsiCaixa Foundation, UAB, Barcelona, Spain; 5Dr J Fourie Medical Centre, Dundee, South Africa; 6Instituto de Pesquisa Clinica Evandro Chagas-Fiocruz, Rio de Janeiro, Brazil; 7Beijing You'an Hospital, China; 8Royal Free Hospital, London, UK; 9Univ Alabama at Birmingham/Infect Dis, Birmingham, USA; 10Section of Infect Dis, Chiang Mai University, Thailand; 11Tibotec BVBA, Beerse, Belgium; 12Tibotec Inc., Titusville, NJ, USA
At least possibly related to treatment
1Mathias A et al. XVIIIth IAC 2010; Abstract LBPE17
1Azijn H, et al. AAC 2010;54:718-27
2Desmidt M, et al. EACS 2009. Abstract PE7.1/4
3Goebel F, et al. AIDS 2006;20:1721-6
4Pozniak A, et al. AIDS. 2010;24:55-65
From 39 NNRTI RAMs based on list of 441
Determined using vircoTYPE HIV-1 test
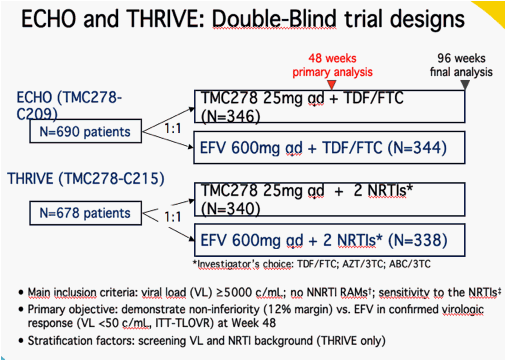
ITT = intent-to-treat; TLOVR = time-to-loss of virologic response
Pooled analyses were preplanned
1Tambuyzer L et al. Antivir Ther 2009;14:103-9
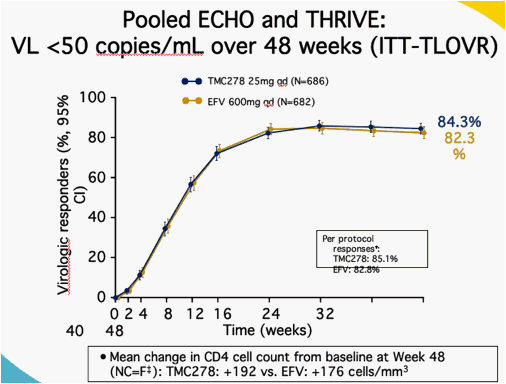
CI = confidence interval; Excluding major protocol violators; missing
values after discontinuation imputed with change = 0; LOCF otherwise
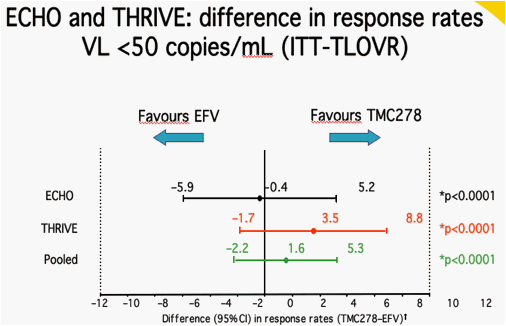
*p-value for non-inferiority at 12% margin; Estimated by logistic regression adjusted for stratification factors
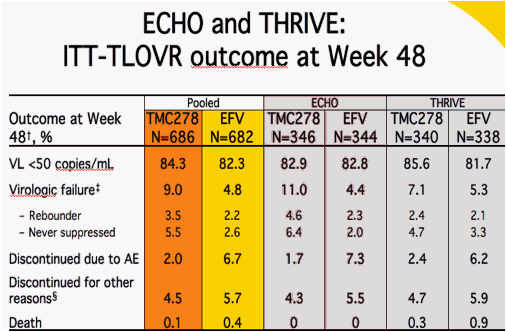
Analysis performed up to Week 48; Determined by TLOVR in the ITT population: confirmed response before Week 48 and confirmed rebound (rebounders) at or before Week 48, or no confirmed response before Week 48 (never suppressed); Lost to follow-up, non-compliance, withdrew consent, ineligible to continue, sponsor's decision; AE = adverse event
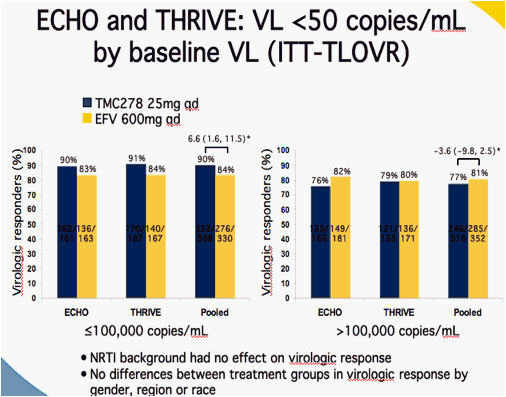
*Difference in response rates (95% CI)
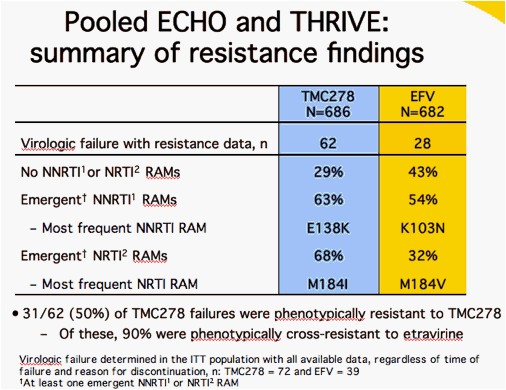
1Tambuyzer L et al. Antivir Ther 2009;14:103-9
2Johnson VA et al. Top HIV Med 2009;17:138-45
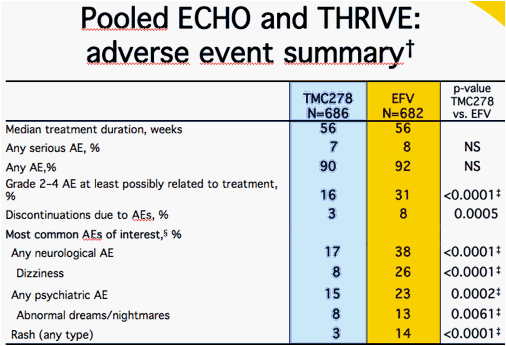
NS = non significant; Safety analyses performed using all available data, including beyond Week 48; Fisher's Exact test, predefined analysis for these AEs; Well-described AEs associated with current NNRTIs at least possibly related to treatment and observed in ≥10% of patients in either group (all grades)
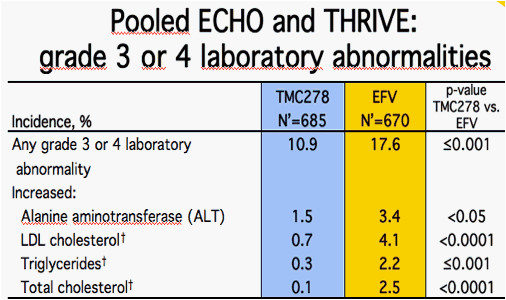
Worst grade, treatment-emergent events occurring in ≥2% of patients in either group and showing statistically significant differences between treatment groups by Fisher's Exact test, post-hoc analyses; N' = number with available test results; Lipid samples taken fasting
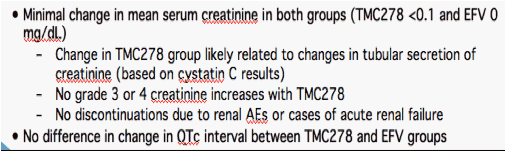
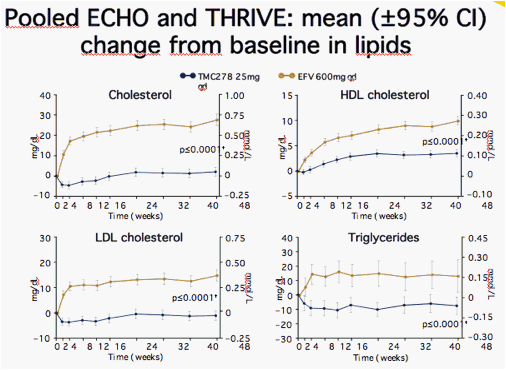
· No difference between groups in total cholesterol/HDL-C ratio at Week 48
· p value vs. EFV at Week 48 (non-parametric Wilcoxon rank-sum test)
|
| |
|
 |
 |
|
|