 |
 |
 |
| |
The Monet Trial 96 Weeks: drunavir Monotherapy vs darunavir + 2 NRTIs inpatients with HIV RNA < 50 copies/ml at baseline
|
| |
| |
Reported by Jules Levin

Monotherapy Durable in MONET Trial
MedPage Today
Published: July 23, 2010
Action Points
* Explain to interested patients that this long-running study suggests that taking a single potent drug can keep HIV under control in some patients.
* Note that this study was published as an abstract and presented at a conference. These data and conclusions should be considered to be preliminary until published in a peer-reviewed journal.
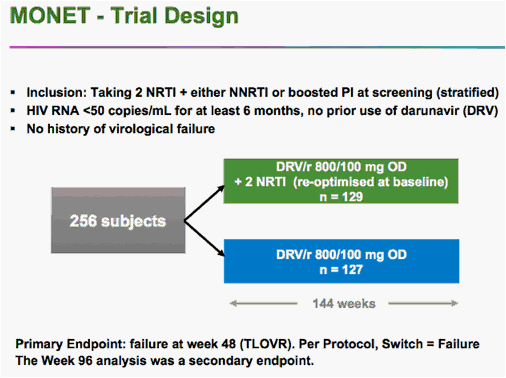
VIENNA -- Switching to monotherapy with the protease inhibitor darunavir (Prezista) appears to be effective for almost two years among patients who start with well-controlled HIV on combination antiretroviral therapy, a researcher said here.
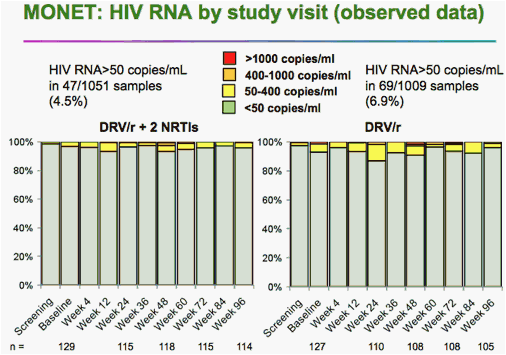
After 96 weeks of darunavir monotherapy, up to 92% of patients still had fewer than 50 copies of viral RNA per milliliter of blood, according to Armin Rieger, MD, of the University of Vienna.
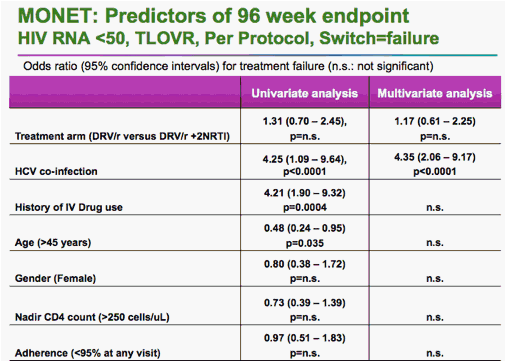
But he told a late-breaker session at the International AIDS Conference that -- using a slightly more restrictive analysis -- monotherapy was statistically inferior to combination therapy with darunavir and two non-nucleoside reverse transcriptase inhibitors.
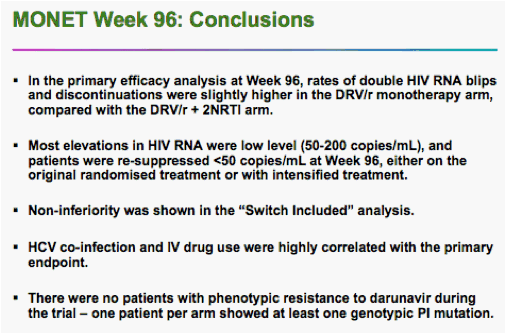
The findings come from the long-running MONET trial, whose main result was that after a year, switching to monotherapy was equivalent to continued combination treatment. The 96-week results were a secondary endpoint of the original trial.
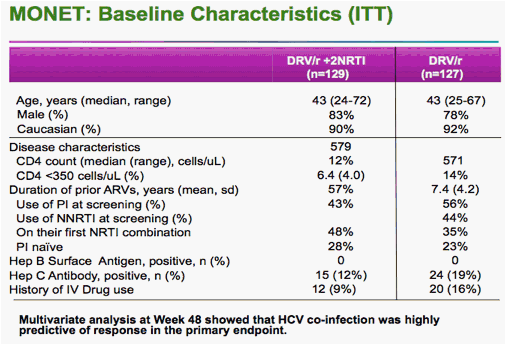
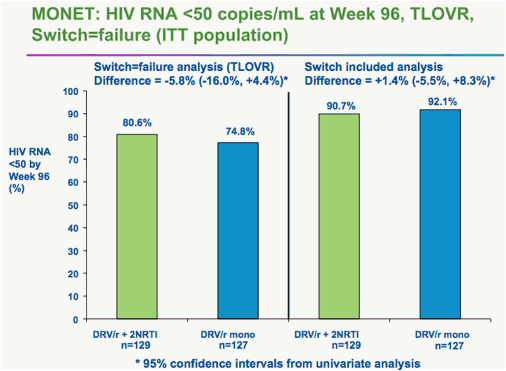
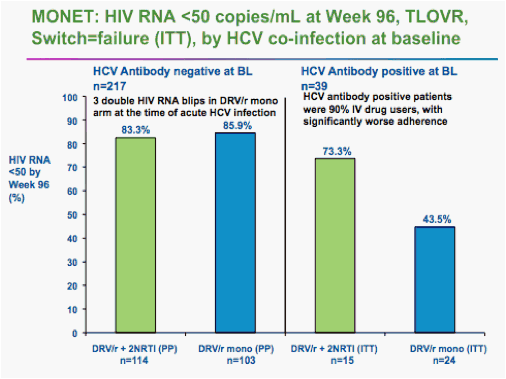
Rieger said he and colleagues analyzed the data after 96 weeks in two ways. In one analysis, two "blips" in HIV viral load to above the 50-copy level counted as a failure, even if the virus was resuppressed to below 50 copies by week 96.
Using that analysis, he said, 74.8% of the monotherapy patients remained suppressed, compared with 80.6% of the triple-drug patients. But the 5.8% difference had a 95% confidence interval that ranged from -16% to 4.4% -- greater than the 12% preset margin for noninferiority.
On that analysis, Rieger said, the monotherapy failed to match triple therapy.
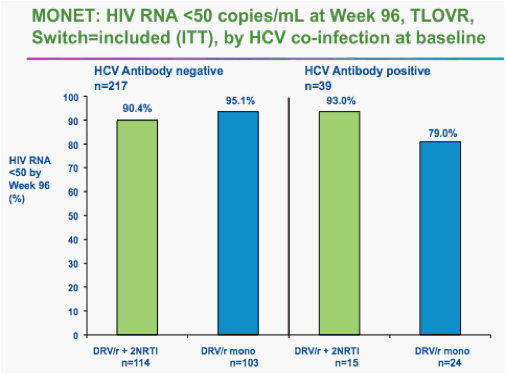
On the other hand, he said, if analysis included those whose HIV was fully suppressed at 96 weeks, regardless of blips along the way, 92.1% of monotherapy patients were still controlling their HIV, compared with 90.7% of the triple-drug patients.
That analysis showed non-inferiority of the two regimens, he said.
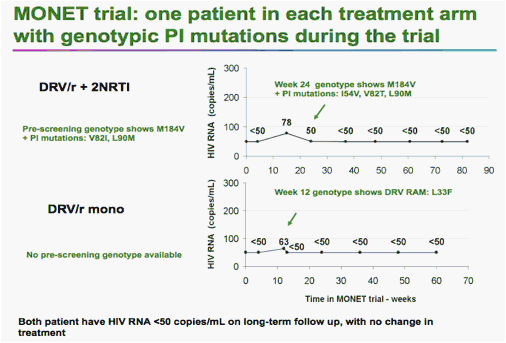
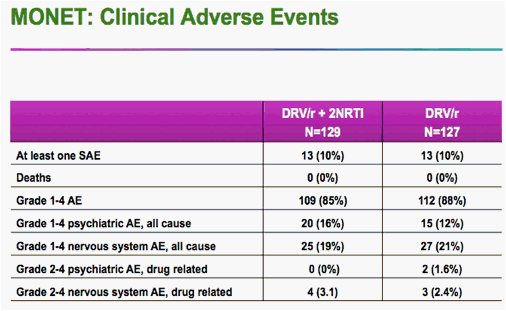
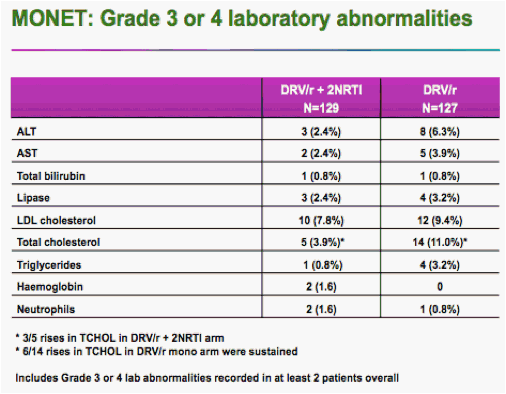
There were two cases of genotypic resistance to darunavir -- one in each arm -- but neither patient showed a physical effect of the mutations, Rieger said.
Monotherapy is an interesting concept with some obvious advantages, including lower drug costs, said Anton Pozniak, MD, of the Westminster and Chelsea Hospital in London, who was not part of the study but moderated the session at which it was presented.
The MONET study seems to show that it can be almost as effective as triple-drug therapy over a long period, provided patients have well-controlled virus at the start, he said.
"It might be that if you're suppressed for years, then you can simplify your regimen," he told MedPage Today after the session.
"We need to be careful with simplification," said Stefano Vella, MD, of the Istituto Superiore di Sanità in Rome, the Italian equivalent of the NIH. Vella, a former president of the AIDS society, was not part of the study.
The idea is to make regimens easier to follow and less costly, he told MedPage Today, but such simplified regimens can easily lead to loss of efficacy and the subsequent rise of resistance.
On the other hand, he echoed Pozniak's comments: "Once you have the virus suppressed, it's easier to keep it down," he said.

|
| |
|
 |
 |
|
|