 |
 |
 |
| |
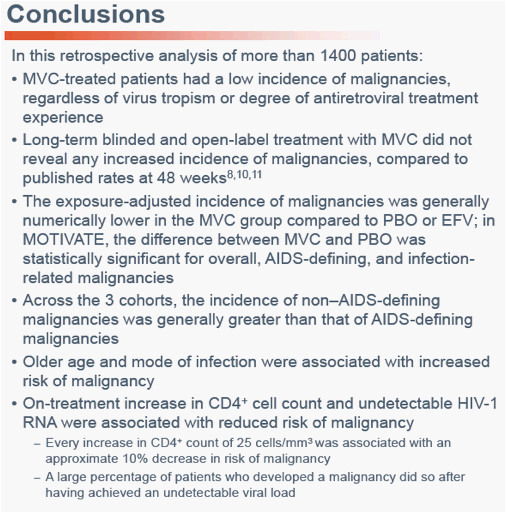
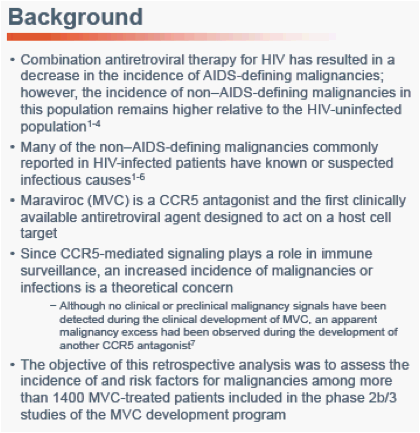
Low Risk of Malignancy With Maraviroc in Treatment-Experienced and Treatment-Naïve Patients Across the Maraviroc Clinical Development Program
|
| |
| |
Reported by Jules Levin, 18th Intl AIDS Conf July 18-23 2010, Vienna Austria
Sharon Walmsley1, Rafael Campo2, James Goodrich3, Jayvant Heera4, Robert Burnside4, Simon Portsmouth5, Hernan Valdez51University of Toronto, Toronto, Ontario, Canada; 2University of Miami, Miami, FL, USA; 3ViiV Healthcare, Research Triangle Park, NC, USA; 4Pfizer Global Research and Development, New London, CT, USA; 5Pfizer Inc, New York, NY, USA
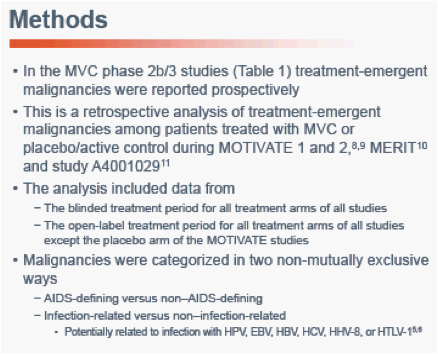
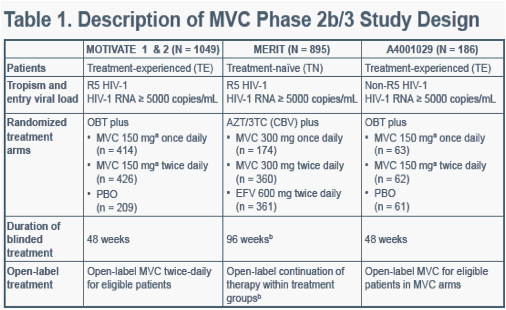
3TC, lamivudine; AZT, zidovudine; CBV, Combivir; EFV, efavirenz; OBT, optimized background therapy; PBO, placeboaPatients receiving protease inhibitors (except tipranavir) and/or delavirdine received 150 mg MVC, all othersreceived 300 mg.
bThe MVC once-daily arm was discontinued at 16 weeks following the results of an interim analysis. Patients in this arm were subsequently eligible to receive open-label MVC 300 mg twice-daily.
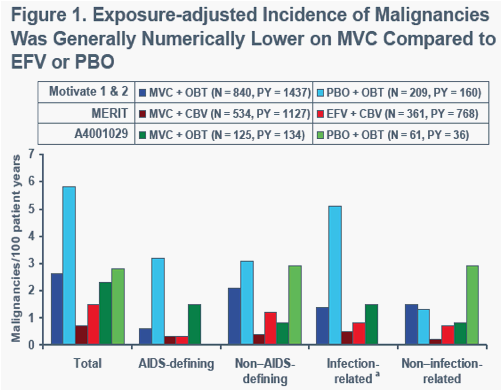
CBV, combivir; EFV, efavirenz; OBT, optimized background therapy; PBO, placebo; PY, patient year
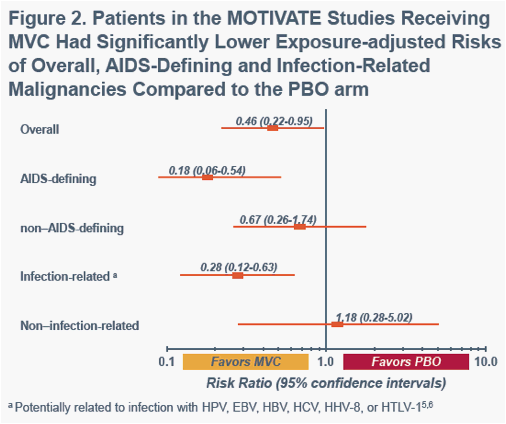
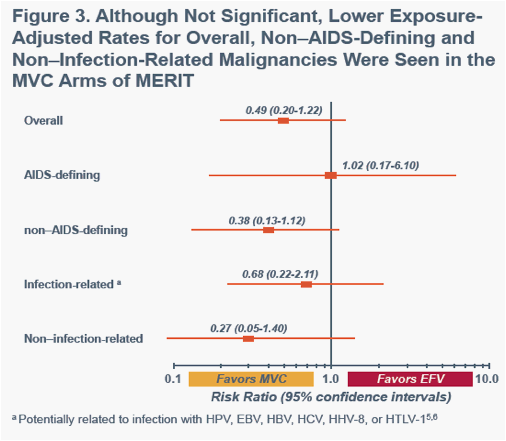
aPotentially related to infection with HPV, EBV, HBV, HCV, HHV-8, or HTLV-15,6a
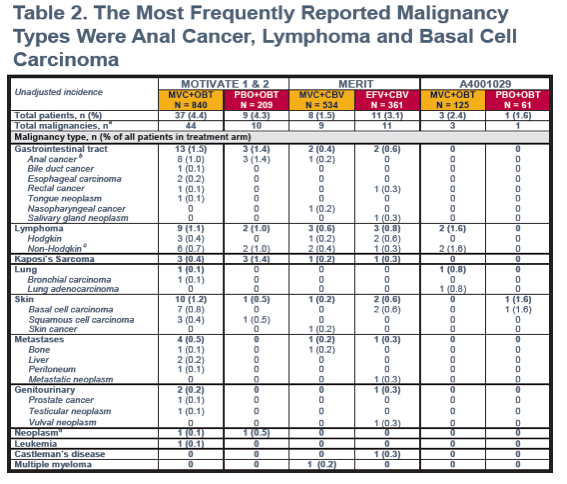
CBV, Combivir; EFV, efavirenz; MVC, Maraviroc; OBT, optimized background therapy.
aAll on-study malignancy events are listed for patients who had one or more malignancy event on study.
bIncludes anal cancer stage 0.
cIncludes Burkitt's lymphoma, T-cell lymphoma, B-cell lymphoma, diffuse large B-cell lymphoma, lymphoma, and other non-Hodgkin lymphoma.
dInvestigator terms "Lump left side neck" and "Right thigh lump" reported under the MedDRA preferred term of neoplasm. Further investigation into the clinical status of these neoplasms is ongoing.
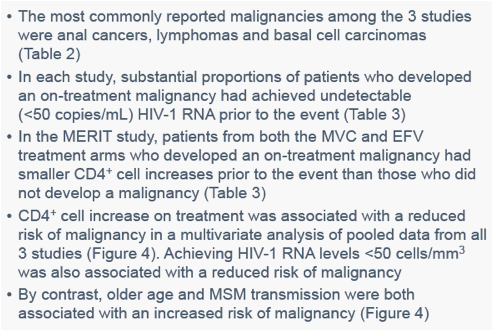
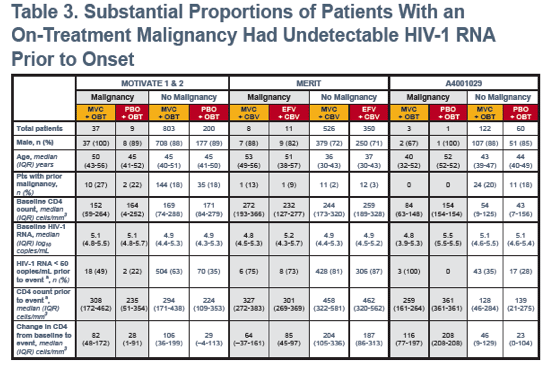
CBV, combivir; EFV, efavirenz; IQR, interquartile range; OBT, optimized background therapy; PBO, placebo.aFor patients without malignancies, listed values are the last on-treatment measurement.
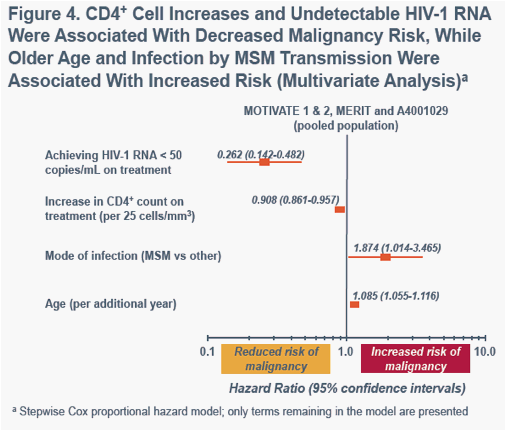
REFERENCES
1.Bedimo RJ, et al. J Acquir Immune Defic Syndr. 2009;52:203-208.
2.Crum-Cianflone N, et al. AIDS. 2009;23:41-50.
3.Pantanowitz L, et al. Curr Opin. HIV AIDS 2009;4:27-34.
4.Bonnet F, Chene G. Curr Opin. Oncol 2008;20:534-540.
5.Grulich AE, et al. Lancet. 2007;370:59-67.
6.Parkin DM. Int J Cancer. 2006;118: 3030-3044.
7.Gulick RM, et al. J Infect Dis. 2007;196:304-312.
8.Gulick RM, et al. N Engl J Med. 2008; 359:1429-1441.
9.Hardy WD, et al. 9th International Congress on Drug Therapy in HIV infection, Glasgow, UK, November 9-13, 2008; Abstract O425.
10.Cooper DA, et al. J Infect Dis. 2010;201: 803-813.11.Saag M, et al. J Infect Dis. 2009;199:1638-1647.
|
| |
|
 |
 |
|
|