 |
 |
 |
| |
Bioequivalence of the Co-Formulation
of Emtricitabine/Rilpivirine/Tenofovir DF
|
| |
| |
Reported by Jules Levin
XVIII International AIDS Conference
July 18-23, 2010
Vienna, Austria
A Mathias, M Menning, X Wei, A Dave, S Chuck and BP Kearney
Gilead Sciences, Inc., Foster City, CA, USA
INTRODUCTION
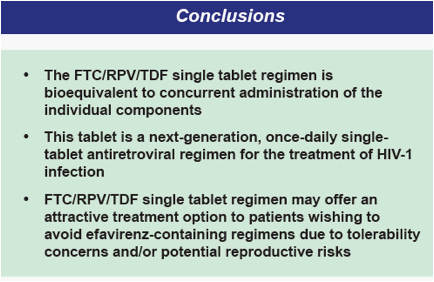
Clinical studies have demonstrated that single tablet HAART regimens lead to high levels of adherence and patient preference, resulting in durable suppression of HIV-1 RNA and improved clinical outcomes1,2
The availability of Efavirenz/Emtricitabine/Tenofovir DF (Atripla®) single tablet regimen as well as other combination tablets has enabled simplifi cation of HIV treatment
There still remains a need for new single tablet regimens composed of potent agents exhibiting favorable tolerability, minimal short and long-term toxicity, and convenient dosing to maximize patient adherence
Rilpivirine (RPV, TMC278) 25mg QD has demonstrated, in a Phase 2b study, efficacy similar to efavirenz with an improved safety profi le with respect to CNS adverse events, lipid abnormalities, incidence of rash and is not teratogenic3
RPV is under evaluation in Phase 3 clinical trials in treatment-naïve HIV-1 patients in combination with NRTI backbone agents including, DHHS- and EACS-preferred emtricitabine (FTC 200mg) and tenofovir disoproxil fumarate (TDF 300mg)
- Refer to Abstract THLBB206 for summary of 48-week effi cacy and safety data from RPV Phase 3 studies
Gilead has co-formulated RPV and the standard-of-care NRTI backbone FTC/TDF into a single tablet regimen
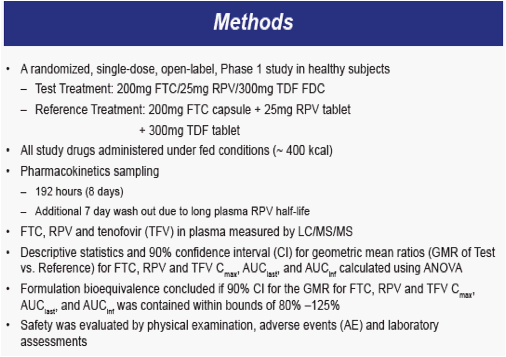
Bioequivalence Study Objectives
To evaluate the pharmacokinetics and bioequivalence of a fixed-dose combination tablet (FDC)
- 200mg FTC/25mg RPV/300mg TDF
- Compared to individual components FTC + RPV + TDF
To assess the safety of FTC, RPV and TDF administered as single tablet regimen and coadministered as individual dosage forms in healthy subjects
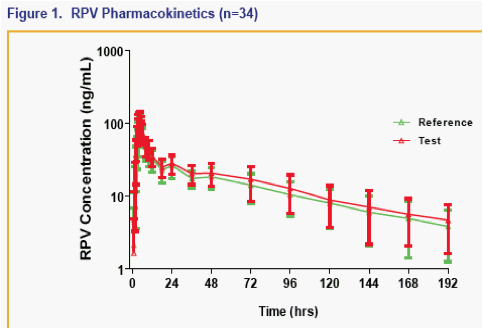
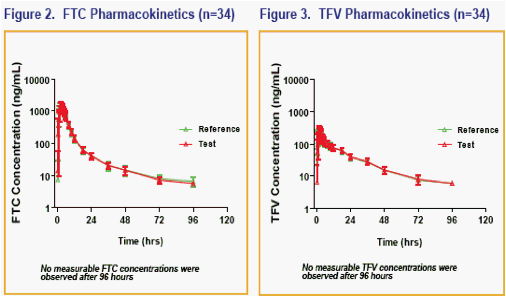
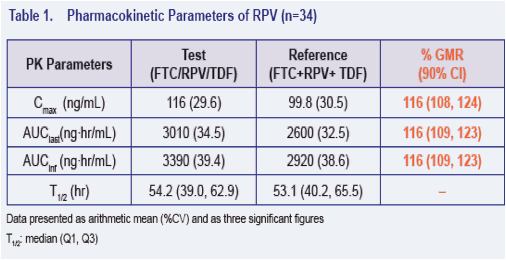
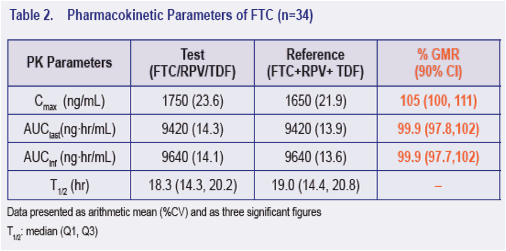
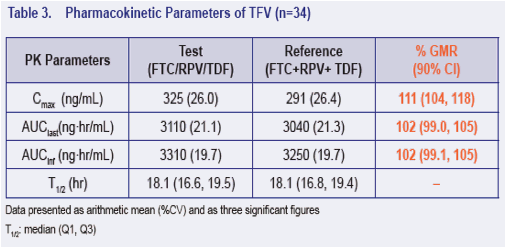
· The 90% CI for the ratio of the geometric least-squares means of Cmax, AUClast and AUCinf for the single tablet regimen versus the individual dosage forms were contained within 80% to 125% for FTC, RPV and TFV
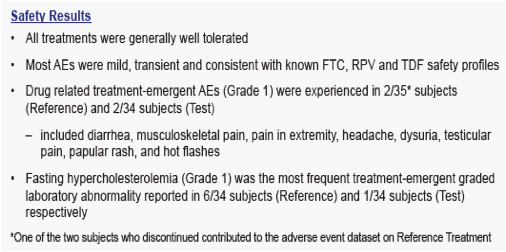
References
1. Airoldi M, et al. " One-pill once-a-day HAART: a simplifi cation strategy that improves adherence and quality of life of HIV-infected subjects" Patient Preference Adherence 2010; 4:115-125.
2. Kitahata MM, et al. "Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death." Intl J STD AIDS 2004; 15:803-810.
3. Pozniak AL, et al. "Effi cacy and safety of TMC278 in antiretroviral-naive HIV-1 patients: week 96 results of a phase IIb randomized trial." AIDS 2010; Jan 2; 24(1):55-65.
|
| |
|
 |
 |
|
|