 |
 |
 |
| |
Antiviral Activity/Resistance Monitoring of HCV Patients Treated for Three Days with the NS5A Inhibitor PPI-461 Reveals Rapid Emergence of Resistant HCV Variants
|
| |
| |
Reported by jules Levin AASLD, the Liver Meeting, Nov 5-9 2011 San Francisco
Qi Huang1, Ningwu Huang1, Miriam Lau1, Margaret Bencsik1, Anja Huq1, Eric Peng1, Kosh Agarwal2, Jay Lalezari3, Pam Vig1, Nathaniel Brown1 and Richard Colonno1
1Presidio Pharmaceuticals, San Francisco, CA, USA, 2Kings College Hospital, London, UK and 3Quest Clinical Research and UCSF, San Francisco, CA, USA
IntroductionPopulation Phenotypes Improved Potency and Lead
Abstract (updated)
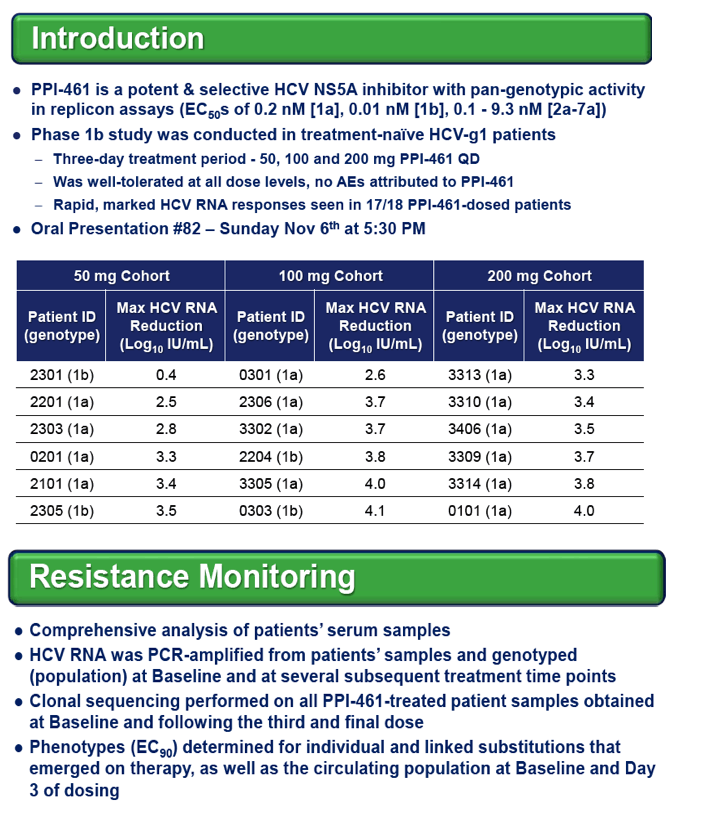
BACKGROUND: PPI-461, a novel potent inhibitor of HCV NS5A, was recently assessed in a Phase 1b 3-day monotherapyclinical trial in HCV genotype-1 patients with oral doses of 50, 100 and 200 mg QD. Mean maximal viral load reductions of 3.6 log10IU/mL were observed in the first 30-48 hr with the two higher doses. This study monitored for resistant HCV variants over the dosing and post-dosing periods of the trial.
METHODS:Eight patients were enrolled per dose cohort (randomized 6:2, active vs. placebo). HCV RNA was extracted from patient plasma samples, PCR amplified and the resulting DNA first subjected to population sequencing, and then clonalsequencing to determine if substitutions were genetically linked. The PPI-461 susceptibility of observed substitutions was assessed in transient transfection assays using HCV replicons encoding either specific substitutions or pooled inserts from clinical samples.
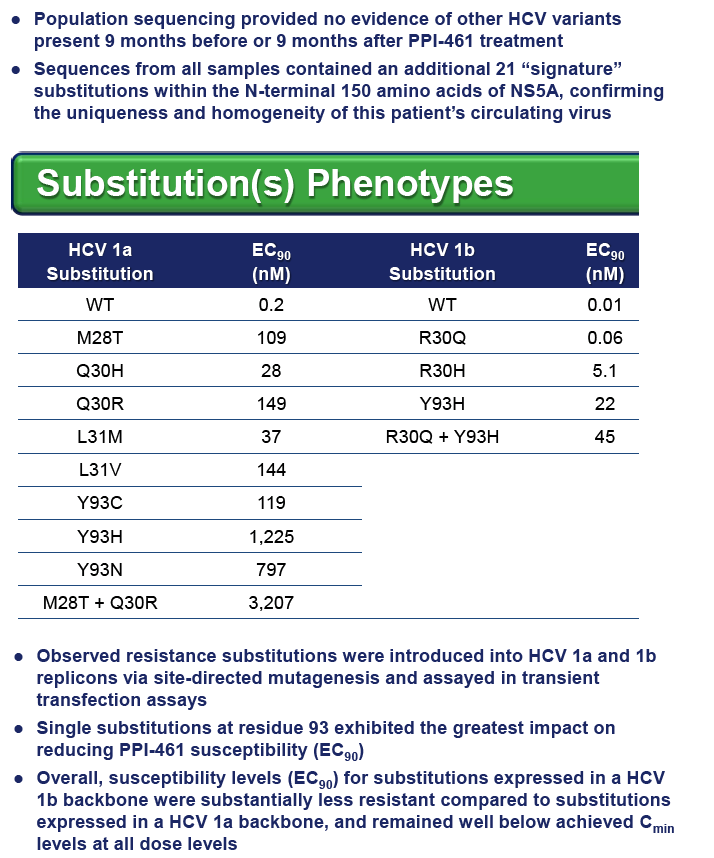
RESULTS: Substitutions at residues 28, 30, 31 and 93 within Domain 1 of the NS5A protein were previously found to be primary resistance substitutions for PPI-461 and other NS5A inhibitors.
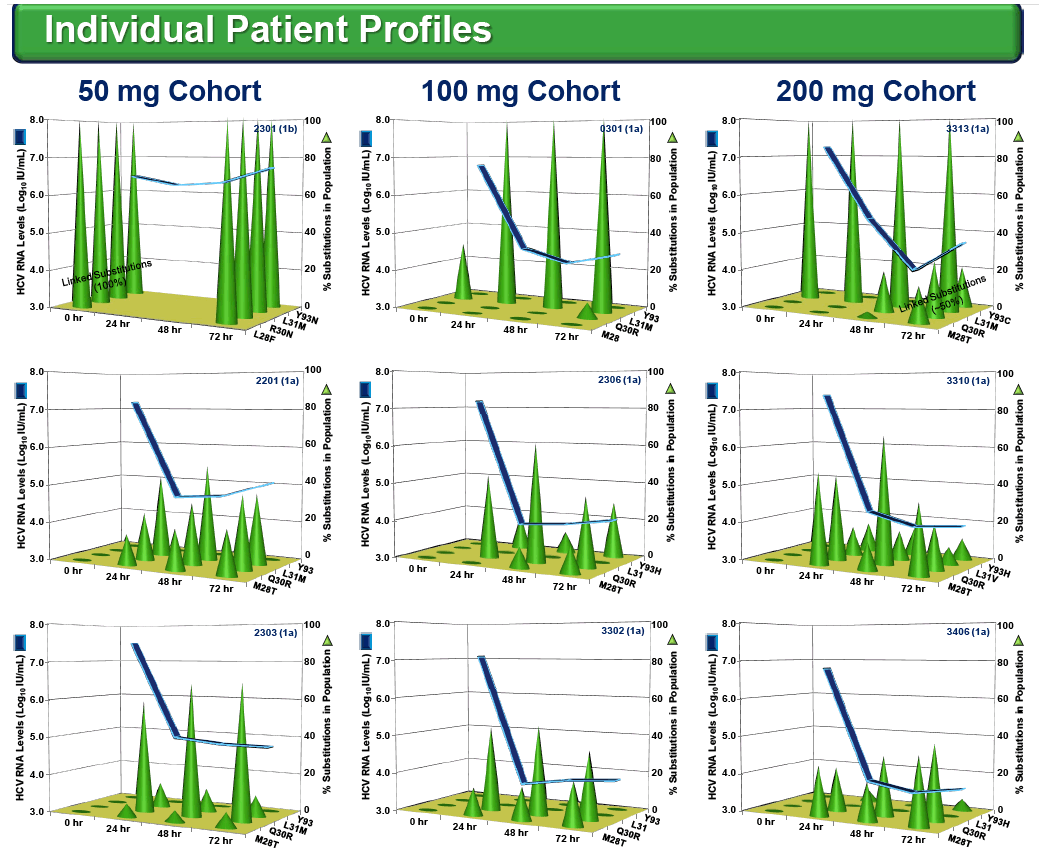
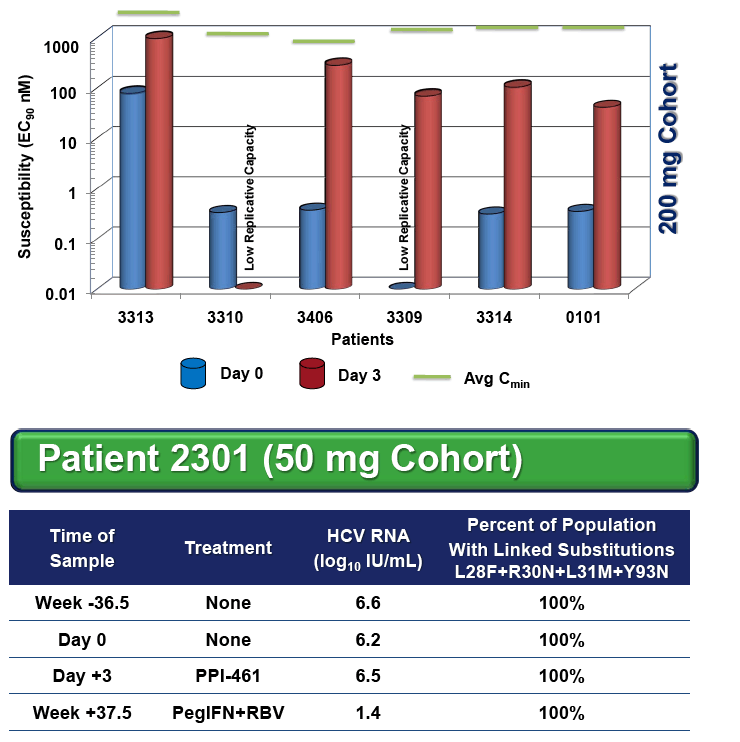
Among the 24 enrolled patients, 5 had either a R30Q substitution (n=2, g1b), a L31M substitution (n=2, g1a) or the linked combination of L28F+R30N+L31M+Y93N (n=1, g1b) in their pre-treatment (Baseline) sera.
All four patients with either a single R30Q or L31M substitution responded well, with viral load reductions of 2.6 to 4.1 log10IU/mL on PPI-461 treatment, whereas the patient with 100% of circulating virus containing all four linked substitutions showed a minimal (0.4 log10) drop, and there was no evidence of wild-type (WT) HCV RNA in samples taken 9 months before and after PPI-461 treatment.
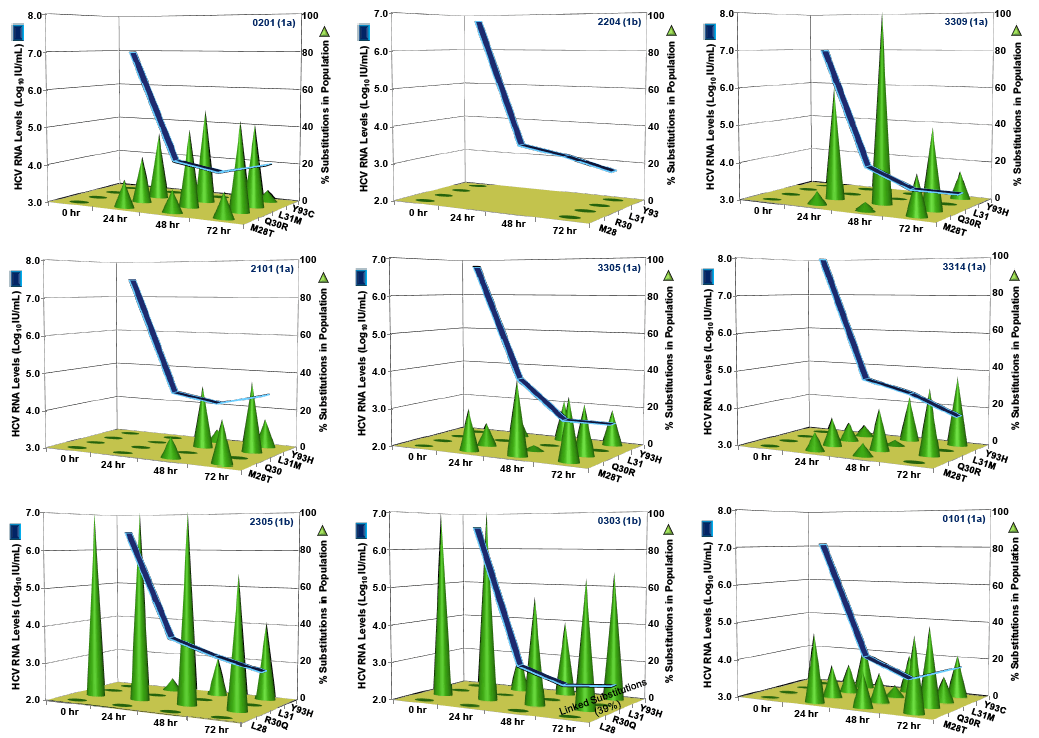
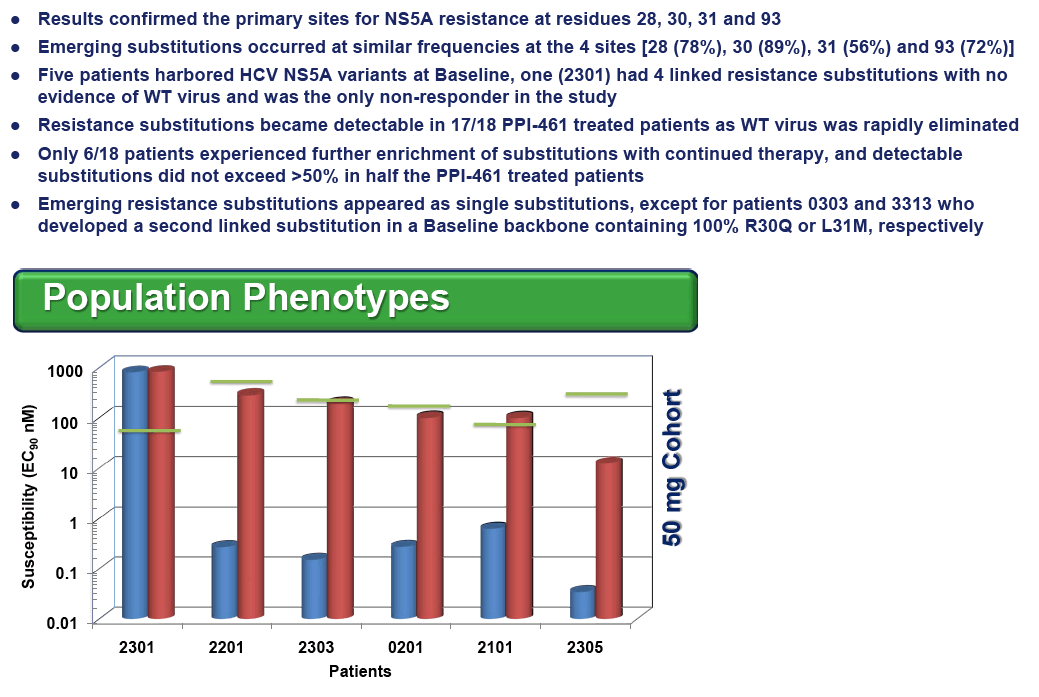
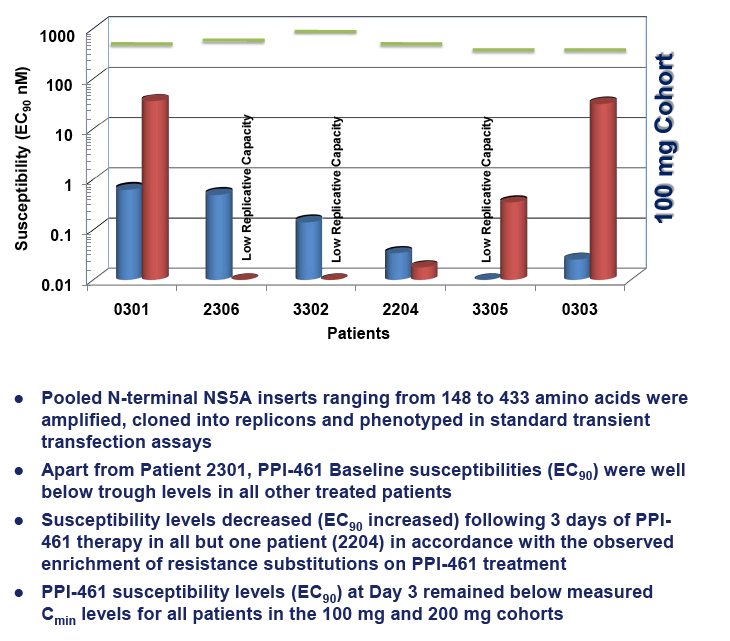
Resistance substitutions M28T, Q30R, L31M and Y93H/C emerged by Day 3 in 13,13, 6 and 12 of the 18 PPI-461 treated patients, respectively. The vast majority of the emerging substitutions were not linked, instead appearing to be distinct viral variants enriched on treatment, some detected as early as 6 hr. Replicon susceptibility (EC90) for pooled samples from five of six patients dosed with 50 mg of PPI-461 reached or exceeded the minimal plasma concentrations (Cmin), while all of the Day 3 phenotypes of pooled samples in the 100 mg and 200 mg cohorts remained below Cminlevels.
CONCLUSIONS: Resistance substitutions were evident in 17 of 18 patients treated with PPI-461 monotherapyfor 3 days, with most patients having only single amino acid substitutions detectable by Day 3. Similar to other classes of HCV antivirals, combination therapy will be required to circumvent the emergence of viral resistance to HCV NS5A inhibitors.
|
| |
|
 |
 |
|
|